

Chinese Journal of Organic Chemistry >
Design, Synthesis and Structure-Activity Relationship ofTryptanthrins as Antitumor Agents
Received date: 2015-07-15
Revised date: 2015-09-14
Online published: 2015-09-25
Supported by
Project supported by the Shaanxi Provincial Natural Science Fundation (No. 2014JM4095), the Foundation of the Education Department of Shaanxi Province (No. 12JK1010), the Key Program for Science and Technology Innovative Research Team of Shaanxi Province (No. 2013KCT-24).
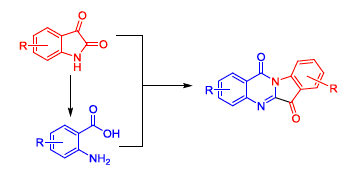
The isatin derivatives 4a~4f were prepared and underwent oxidative hydrolysis to give the anthranilic acid 5a~5d. A and/or D-ring substituted tryptanthrins were designed and synthesized from 4a~4f to 5a~5d. Then C-ring Schiff bases of tryptanthrin were synthesized by condensation of 6-carbonyl with hydrazine and hydroxylamine hydrochloride. Finally, the B-ring was replaced with piperazine to give 11H-indeno[1,2-b]quinoxalin-11-one. 20 compounds were synthesized and their structures were confirmed by 1H NMR, IR and elemental analysis. To best of our knowledge, 13 of them were unknown in the literature. The antitumor activities of synthesized compounds were evaluated against A549 cell line in vitro. The preliminary results indicated that 1b, 1c, 1i, 1j, 1p and 1q showed good antitumor activity with the IC50 of 3.58, 0.99, 1.03, 2.10, 0.51 and 0.43 μmol·L-1, respectively. Structure-activity relationship showed that halogen substitution located in the D-ring enhanced the anti-tumor activity, while the same substitution located in the A ring reduced the activity. The anti-tumor activity disappeared when B-ring was replaced by piperazine, while there was no significant difference for tryptanthrin and its C-ring Schiff base.
Key words: tryptanthrin; isatin; anthranilic acid; antitumor activity
Hou Baolong , Ai Yun , Wang Cuiling , Zhang Ning , Yang Liu , Liu Zhulan , Liu Jianli . Design, Synthesis and Structure-Activity Relationship ofTryptanthrins as Antitumor Agents[J]. Chinese Journal of Organic Chemistry, 2016 , 36(1) : 121 -129 . DOI: 10.6023/cjoc201507012
[1] Honda, G.; Tabata, M. Planta Med. 1979, 36, 85.
[2] Honda, G.; Tosirisuk, V.; Tabata, M. Planta Med. 1980, 38, 275.
[3] Henning, D.; Dietmar, B.; Matthias, H. Planta Med. 2002, 68, 152.
[4] Schindler, W.; Zähner, H. Arch. Mikrobiol1971, 79, 187.
[5] Yurngdong, J. Arch. Pharm. Res. 2013, 36, 517.
[6] Bandekar, P. P.; Roopnarine, K. A.; Parekh, V. J.; Mitchell, T. R.; Novak, M. J.; Sinden, R. R. J. Med. Chem. 2010, 53, 3558.
[7] Takel, Y.; Kunikata, T.; Aga, M.; Inoue, S.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. J. Biol. Pharm. Bull. 2003, 26, 365.
[8] Taterao, M.; Sachin, P.; Kumar, A.; Srinivasan, V. I. ARKIVOC 2008, xiv, 100.
[9] Bhattacharjee, A. K.; Skanchy, D. J.; Jennings, B.; Hudson, T. H.; Brendle, J. J.; Werbovetz, K. A. Bioorg. Med. Chem. 2002, 10, 1979.
[10] Sharma, V. M.; Prasanna, P.; Seshu, K. V. A.; Renuka, B.; Rao, C. V. L.; Kumar, G. S.; Narasimhulu, C. P.; Babu, P. A.; Puranik, R. C.; Subramanyam, D.; Venkateswarlu, A.; Rajagopal, S.; Kumar, K. B. S.; Rao, C. S.; Mamidi, N. V. S. R.; Deevi, D. S.; Ajaykumar, R.; Rajagopalan, R. Bioorg. Med. Chem. Lett. 2002, 12(17), 2303.
[11] Yu, S. T.; Chern, J. W.; Chen, T. M.; Chiu, Y. F.; Chen, H. T.; Chen, Y. H. Acta Pharmacol. Sin. 2010, 31, 259.
[12] Pathnia, A. S.; Kumar, S.; Guru, S. K.; Bhushan, S.; Sharma, P. R.; Aithagani, S. K.; Singh, P. P.; Vishwakarma, R. A.; Kumar, A.; Malik, F. PLoS One 2014, 9, e110411.
[13] Hwang, J. M.; Oh, T.; Kaneko, T.; Upton, A. M.; Franzblau, S. G.; Ma, Z.; Cho, S. N.; Kim, P. J. Nat. Prod. 2013, 76, 354.
[14] Pergola, C.; Jazzar, B.; Rossi, A.; Northoff, H.; Hamburger, M.; Sautebin, L.; Werz, O. Br. J. Pharmacol. 2012, 165, 765.
[15] Friedlander, N.; Roschdestwensky, P. Chem. Ber. 1915, 48, 1841.
[16] Eguehi, S. ARKIVOC 2005, 11, 98.
[17] Bergman, J.; Lindstrm, J. O.; Tilstam, U. Tetrahedron 1985, 41, 2879.
[18] Yu, S. T.; Chern, J. W.; Chen, T. M.; Chiu, Y. F.; Chen, H. T.; Chen, Y. H. Acta Pharmacol. Sin. 2010, 31, 259.
[19] Wang, C. L.; Hou, B. L.; Zhang, N.; Sun, Y. N.; Liu, J. L. Chem. J. Chin. Univ.2015, 36, 274 (in Chinese).(王翠玲, 侯宝龙, 张宁, 孙艳妮, 刘建利, 高等学校化学学报, 2015, 36, 274.)
[20] Wang, Z.; Wang, C. L.; Sun, Y. N.; Zhang, N.; Liu, Z. L.; Liu, J. L. Tetrahedron 2014, 70.
[21] Gao, W. T.; Zhao, P. B.; Zhao, B. B.; Li, Y. Chin. J. Org. Chem. 2014, 34, 126 (in Chinese).(高文涛, 赵鹏波, 赵宾宾, 李阳, 有机化学, 2014, 34, 126.)
[22] Wang, C. L.; Liu, Z. L.; Hou, B. L.; Wang, J.; Zhang, N.; Liu, J. L. J. Northwest Univ.(Nat. Sci. Ed.) 2011, 41, 817 (in Chinese).(王翠玲, 刘竹兰, 侯宝龙, 王瑾, 张宁, 刘建利, 西北大学学报(自然科学版), 2011, 41, 817.)
[23] Stiff, C.; Graber, D. R.; Thorarensen, A.; Wakefield, B. D.; Marotti, K. R.; Melchior, E. P.; Sweeney, M. T.; Han, F.; Rohrer, D. C.; Zurenkoc, G. E.; Romeroa, D. L. Bioorg. Med. Chem. Lett. 2008, 18, 6293.
[24] Zhao, Y.; Ouyang, G. P.; Xu, W.M.; Jin, L.H.; Yuan, K. Chin. J. Org. Chem. 2010, 30, 1093 (in Chinese).(赵云, 欧阳贵平, 徐维民, 金林红, 袁凯, 有机化学, 2010, 30, 1093.)
[25] Zhou, W.; Liu, X. F.; Tu, Z. C.; Zhang, L. W.; K, X.; Bai, F.; Zhao, Z. J.; X, Y. F.; Deng, K.; Li, H. L. J. Med. Chem. 2013, 56, 7821.
[26] Wu, C. H.; Coumar, M. S.; Chu, C. Y.; Lin, W. H.; Chen, Y. R.; Chen, C. T.; Shiao, H. Y.; Rafi, S.; Wang, S. Y.; Hsu, H.; Chen, C. H.; Chang, C. Y.; Chang, T. Y.; Lien, T. W.; Fang, M. Y.; Yeh, K. C.; Chen, C. P.; Yeh, T. K.; Hsieh, S. H.; Hsu, J. T. A.; Liao, C. C.; Chao, Y. S.; Hsieh, H. P. J. Med. Chem. 2010, 53, 7316.
[27] Chen, Y.; Bai, S.; He, H. W.; Yang, G. Z. Chin. J. Org. Chem. 2014, 34, 2362 (in Chinese).(陈玉, 柏舜, 贺红武, 杨光忠, 有机化学, 2014, 34, 2362.)
[28] Zhou, L. H.; Tu, S. J.; Shi, D. Q. J. Chem. Res. 1998, 398.
[29] Andreas, G.; Guenther, S.; Gerhard, W. DE 4114990, 1992 [Chem. Abstr. 1993, 119, 10457].
[30] Bogdana, K.; Amber, C. N.; Kelsi, A. D.; Peter, G. Bioorg. Med. Chem. Lett. 2013, 23, 1032.
[31] Manickam, B.; Raman, S.; Jayakumar, S. Tetrahedron Lett. 2014, 55, 5808.
/
| 〈 |
|
〉 |