

Chinese Journal of Organic Chemistry >
Application of Bifunctional (Thio)ureas with Auxiliary in Asymmetric Organocatalysis
Received date: 2015-08-30
Revised date: 2015-09-18
Online published: 2015-10-08
Supported by
Project supported by the National Natural Science Foundation of China (No. 20906029)
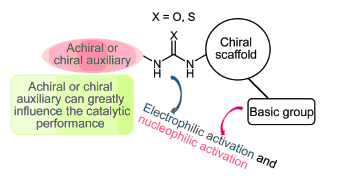
Chiral bifunctional (thio)ureas, which are made up of (thio)urea group, nucleophilic activation group and auxiliary, have attracted widespread attention in asymmetric organocatalysis research nowadays because of their easy control structure and excellent catalytic performance. The introduction of the activation group, which can greatly influence the catalytic performance of the catalyst, has become an important and concern research field for the catalyst design, while the introduction of suitable auxiliary can also be used to control and optimize catalytic performance, and become an important supplementary method. However, up to now, there is no systematical review exclusively on utilization of auxiliary strategy for the construction of chiral bifunctional (thio)ureas. In this paper, the research progress of the construction of chiral bifunctional (thio)ureas based on tunable achiral and chiral auxiliaries in recent years is reviewed. These catalysts can be successfully applied in a diverse variety of highly enantioselective transformations providing a wide range of versatile organic compounds. The influence of several factors in the auxiliaries, such as steric hindrance, chiral environment, electronic effect and hydrogen bond donor, on the catalytic performance is described. Besides, an outlook for future development of auxiliaries to construct bifunctional (thio)ureas is given.
Chen Xuewei , Xin Yujuan , Liu Qing , Lan Zhili . Application of Bifunctional (Thio)ureas with Auxiliary in Asymmetric Organocatalysis[J]. Chinese Journal of Organic Chemistry, 2016 , 36(2) : 306 -314 . DOI: 10.6023/cjoc201508029
[1] (a) Taylor, M. S.; Jacobsen, E. N. Angew. Chem., Int. Ed. 2006, 45, 1520. (b) Connon, S. J. Chem. Eur. J. 2006, 12, 5418. (c) Doyle, A. G.; Jacobsen, E. N. Chem. Rev. 2007, 107, 5713.
[2] Sigman, M. S.; Jacobsen, E. N. J. Am. Chem. Soc. 1998, 120, 4901.
[3] Okino, T.; Hoashi, Y.; Takemoto, Y. J. Am. Chem. Soc. 2003, 125, 12672.
[4] (a) Wu, Q.-H.; Gao, Y.-J.; Li, Z.; Wang, J.-M.; Wang, C.; Ma, J.-J.; Song, S.-J. Chin. J. Org. Chem. 2007, 27, 1491 (in Chinese). (吴秋华, 高勇军, 李芝, 王俊敏, 王春, 马晶军, 宋双居, 有机化学, 2007, 27, 1491.) (b) Miyabe, H.; Takemoto, Y. Bull. Chem. Soc. Jpn. 2008, 81, 785. (c) Yu, X.; Wang, W. Chem. Asian J. 2008, 3, 516. (d) Takemoto, Y. Chem. Pharm. Bull. 2010, 58, 593. (e) Sohtome, Y.; Nagasawa, K. Synlett 2010, 1. (f) Chai, Z.; Zhao, G. Catal. Sci. Technol. 2012, 2, 29. (g) Lu, L.-Q.; An, X.-L.; Chen, J.-R.; Xiao, W.-J. Synlett 2012, 490. (h) Hou, X.-H.; Ma, Z.-W.; Wang, J.-L.; Liu, H.-M. Chin. J. Org. Chem. 2014, 34, 1509 (in Chinese). (侯学会, 马志伟, 王建玲, 刘宏民, 有机化学, 2014, 34, 1509.)
[5] (a) Connon, S. J. Chem. Commun. 2008, 2499. (b) Siau, W. Y.; Wang, J. Catal. Sci. Technol. 2011, 1, 1298. (c) Tsakos, M.; Kokotos, C. G. Tetrahedron 2013, 69, 10199. (d) Serdyuk, O. V.; Heckel, C. M.; Tsogoeva, S. B. Org. Biomol. Chem. 2013, 11, 7051. (e) Zhang, Z.-G.; Schreiner, P. R. Chem. Soc. Rev. 2009, 38, 1187.
[6] (a) Zhang, Z.-H.; Dong, X.-Q.; Teng, H.-L.; Tao, H.-Y.; Wang, C.-J. Chin. Sci. Bull. 2009, 54, 3407 (in Chinese). (张志海, 董秀琴, 滕怀龙, 陶海燕, 王春江, 科学通报, 2009, 54, 3407.) (b) Fang, X.; Wang, C.-J. Chem. Commun. 2015, 51, 1185. (c) Narayanaperumal, S.; Rivera, D. G.; Silva, R. C.; Paixão, M. W. ChemCatChem 2013, 5, 2756.
[7] Lippert, K. M.; Hof, K.; Gerbig, D.; Ley, D.; Hausmann, H.; Guenther, S.; Schreiner, P. R. Eur. J. Org. Chem. 2012, 5919.
[8] Wei, Q.; Gong, L.-Z. Org. Lett. 2010, 12, 1008.
[9] He, T.; Qian, J.-Y.; Song, H.-L.; Wu, X.-Y. Synlett 2009, 3195.
[10] Ban, S.-R.; Zhu, X.-X.; Zhang, Z.-P.; Li, Q.-S. Eur. J. Org. Chem. 2013, 2977.
[11] Yu, L.; Li, P.-F. Tetrahedron Lett. 2014, 55, 3697.
[12] Li, J.; Yang, G.-X.; Cui, Y.-C. J. Appl. Polym. Sci. 2011, 121, 1506.
[13] Li, J.; Yang, G.-X.; Qin, Y.-Y.; Yang, X.-R.; Cui, Y.-C. Tetrahedron: Asymmetry 2011, 22, 613.
[14] Miura, T.; Nishida, S.; Masuda, A.; Tada, N.; Itoh, A. Tetrahedron Lett. 2011, 52, 4158.
[15] Tuchman-Shukron, L.; Miller, S, J.; Portnoy, M. Chem. Eur. J. 2012, 18, 2290.
[16] (a) Tsogoeva, S.-B.; Wei, S. Chem. Commun. 2006, 1451. (b) Yalalov, D. A.; Tsogoeva, S. B.; Schmatz, S. Adv. Synth. Catal. 2006, 348, 826.
[17] Wang, Y.-F.; Zhang, W.; Luo, S.-P.; Li, B.-L.; Xia, A.-B.; Zhong, A.-G.; Xu, D.-Q. Chem. Asian J. 2009, 4, 1834.
[18] Rao, K. S.; Trivedi, R.; Kantam, M. L. Synlett 2015, 221.
[19] Durmaz, M.; Sirit, A. Tetrahedron: Asymmetry 2013, 24, 1443.
[20] Lalonde, M. P.; Chen, Y.-G.; Jacobsen, E. N. Angew. Chem., Int. Ed. 2006, 45, 6366.
[21] Lalonde, M. P.; McGowan, M. A.; Rajapaksa, N. S.; Jacobsen, E. N. J. Am. Chem. Soc. 2013, 135, 1891.
[22] Kokotos, C. G.; Kokotos, G. Adv. Synth. Catal. 2009, 351, 1355.
[23] Liu, K.; Cui, H.-F.; Nie, J.; Dong, K.-Y.; Li, X.-J.; Ma, J.-A. Org. Lett. 2007, 9, 923.
[24] Lu, A.-D.; Gao, P.; Wu, Y.; Wang, Y.-M.; Zhou, Z.-H.; Tang, C.-C. Org. Biomol. Chem. 2009, 7, 3141.
[25] Pu, X.-W.; Peng, F.-Z.; Zhang, H.-B.; Shao, Z.-H. Tetrahedron 2010, 66, 3655.
[26] Lu, A.-D.; Hu, K.-L.; Wang, Y.-M.; Song, H.-B.; Zhou, Z.-H.; Fang, J.-X.; Tang, C.-C. J. Org. Chem. 2012, 77, 6208.
[27] Jiang, X.-X.; Zhang, Y.-F.; Chan, A. S. C.; Wang, R. Org. Lett. 2009, 11, 153.
[28] Jiang, X.-X.; Cao, Y.-M.; Wang, Y.-Q.; Liu, L.-P.; Shen, F.-F.; Wang, R. J. Am. Chem. Soc. 2010, 132, 15328.
[29] Li, P.-F.; Wang, Y.-C.; Liang, X.-M.; Ye, J.-X. Chem. Commun. 2008, 44, 3302.
[30] Tan, B.; Candeias, N. R.; Barbas, C. F., III Nat. Chem. 2011, 3, 473.
[31] Ma, Z.-W.; Liu, Y.-X.; Zhang, W.-J.; Tao, Y.; Zhu, Y.; Tao, J.-C.; Tang, M. S. Eur. J. Org. Chem. 2011, 6747.
[32] Song, Z.-T.; Zhang, T.; Du, H.-L.; Ma, Z.-W.; Zhang, C.-H.; Tao, J.-C. Chirality 2014, 26, 121.
[33] Tzeng, Z.-H.; Chen, H.-Y.; Huang, C.-T.; Chen, K.-M. Tetrahedron Lett. 2008, 49, 4134.
[34] Wang, C.-J.; Zhang, Z.-H.; Dong, X.-Q.; Xue, Z.-Y.; Teng, H.-L. J. Am. Chem. Soc. 2008, 130, 8606.
[35] Wang, C.-J.; Zhang, Z.-H.; Dong, X.-Q.; Wu, X.-J. Chem. Commun. 2008, 44, 1431.
[36] Jones, C. R.; Panto?, G. D.; Morrison, A. J.; Smith, M. D. Angew. Chem., Int. Ed. 2009, 48, 7391.
[37] Deng, H.-P.; Shi, M. Eur. J. Org. Chem. 2012, 183.
/
| 〈 |
|
〉 |