

Chinese Journal of Organic Chemistry >
A Highly Efficient Paal-Knorr Synthesis of Chiral Pyrrole Derivatives Catalyzed by MgI2
Received date: 2015-05-25
Revised date: 2015-08-17
Online published: 2015-10-13
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21372203, 21272076), the National University Student Innovation Test Plan (No. 201310337007) and the Zhejiang Xinmiao Talent Projects (No. 2014R403021).
A novel methodology has been developed for the synthesis of N-substituted mono-pyrrole and bis-pyrrole derivatives with a chiral substituent at the nitrogen atom by MgI2-catalyzed Paal-Knorr condensation using esters of amino acids as the source of chirality. This method has some advantages such as mild reaction conditions, simple procedure, good yields, high stereoselectivity and environmental friendliness.
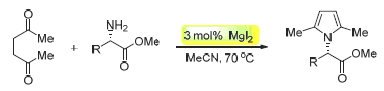
Li Pengcheng , Weng Guodong , Zhang Yongdong , Zhang Xingxian . A Highly Efficient Paal-Knorr Synthesis of Chiral Pyrrole Derivatives Catalyzed by MgI2[J]. Chinese Journal of Organic Chemistry, 2016 , 36(2) : 364 -369 . DOI: 10.6023/cjoc201505041
[1] Demirb, A. S. Tetrahedron 2007, 63, 9746.
[2] Boger, D. L.; Boyce, C. W.; Labrili, M. A.; Sehon, C. A.; Jin, Q. J. Am. Chem. Soc. 1999, 121, 54;
[3] Thirumalairajan, S.; Pearce, B. M.; Thompson, A. Chem. Commun. 2010, 46, 1797.
[4] Ryzhkov, I. O.; Andreev, I. A.; Belov, G. M.; Kurkin, A. V.; Yurovskaya, M. A. Chem. Heterocycl. Compd. 2011, 47, 182.
[5] Wang, Z.-S.; Ye, H.-T. Xiao, D.; Ma, D.-S.; Gao, J.-S.; Yu, Y.-H.; Hou, G.-F.; Yan, P.-F. Chin. J. Org. Chem. 2014, 34, 2057 (in Chinese). (王子时, 叶汉韬, 肖丹, 马东升, 高金胜, 于颖慧, 侯广峰, 闫鹏飞, 有机化学, 2014, 34, 2057.)
[6] Dyatkina, N. B.; Roberts, C. D.; Keicher, J. D.; Joshua, Y. D.; Nadherny, P.; Zhang, W.; Schmitz, U.; Kongpachith, A.; Fung, K.; Novikov, A. A.; Lou, L.; Velligan, M.; Khorlin, A. A.; Chen, M. S. J. Med. Chem. 2002, 45, 805.
[7] Gupton, J. T. Top. Heterocycl. Chem. 2006, 2, 53.
[8] Dieter, R. K.; Yu, H. Org. Lett. 2000, 2, 2283.
[9] Palacios, F.; Aparico, D.; Santos, J. M.; Vicario, J. M. Tetrahedron 2001, 57, 1961.
[10] Berree, F.; Marchand, E.; Morel, G. Tetrahedron Lett. 1992, 33, 6155.
[11] Katritzky, A.; Jiang, J.; Steel, P. J. J. Org. Chem. 1994, 59, 4551.
[12] Katritzky, A.; Steel, P. J. J. Org. Chem. 1994, 59, 4551.
[13] Ramanathan, B.; Keith, A. J.; Armstrong, D.; Odom, A. L. Org. Lett. 2004, 6, 2957.
[14] Iwasawa, N.; Maeyama, K.; Saitou, M. J. Am. Chem. Soc. 1997, 119, 1486.
[15] (a) Hantzsch, A. Chem. Ber. 1890, 23, 1474. (b) Broadbent, H. S.; Burnharm, W. S.; Olsen, R. K.; Sheely, R. M. J. Heterocycl. Chem. 1968, 5, 757. (c) Bayer, H. O.; Gotthardt, H.; Huisgen, R. Chem. Ber. 1970, 103, 2356. (d) Huisgen, R.; Gotthardt, H.; Bayer, H. O.; Schaefter, F. C. Chem. Ber. 1970, 103, 2611. (e) Cooney, J. V.; McEwen, W. E. J. Org. Chem. 1981, 46, 2570. (f) Arcadi, A.; Rossi, E. Tetrahedron 1998, 54, 15253. (g) Periasamy, M.; Srinivas, G.; Bharati, P. J. Org. Chem. 1999, 64, 4204. (h) Srinivas, R.; Thirupathi, B.; Kumar, J. K. Prashanth; Prasad, A. N.; Reddy, B. M. Curr. Org. Chem. 2012, 16, 2482. (i) Yue, M.; Liu, J.; Wang, Y.; Yuan, B. Chin. J. Org. Chem. 2014, 34, 190 (in Chinese). (岳闽敏, 刘巨艳, 王英, 袁斌. 有机化学, 2014, 34, 190.) (j) Yuan, D.; Zeng, M.; Bai, C.; Cui, D.; Zhang, C. Chin. J. Org. Chem. 2014, 34, 1231 (in Chinese). (原东鹏, 曾明, 柏春美, 崔冬梅, 张辰, 有机化学, 2014, 34, 1231.) (k) Wu, C.; Zang, H.; Li, D.; Yu, N.; Cheng, B.; Yu, K. Chin. J. Org. Chem. 2013, 33, 2417 (in Chinese). (吴长春, 臧洪俊, 李大庆, 於年琴, 程博闻, 于楷, 有机化学, 2013, 33, 2417.)
[16] Yu, S. X.; Quesne, P. W. L. Tetrahedron Lett. 1995, 36, 6205.
[17] Rahmatpour, A. Appl. Organomet. Chem. 2011, 25, 585.
[18] Chen, J.; Wu, H.; Zheng, Z.; Jin, C.; Zhang, X.; Su, W. Tetrahedron Lett. 2006, 47, 5383.
[19] Banik, B. K.; Banik, I.; Renteria, M.; Dasgupta, S. K. Tetrahedron Lett. 2004, 45, 3417.
[20] Zhang, Z.-H.; Li, J.-J.; Li, T.-S. Ultrason. Sonochem. 2008, 15, 673.
[21] Aghapoor, K.; Ebadi-Nia, L.; Mohsenzadeh, F. J. Organomet. Chem. 2012, 708, 25.
[22] Shanthi, G.; Perumal, P. T. Tetrahedron Lett. 2009, 50, 3959.
[23] Azizi, N.; Khajeh-Amiri, A.; Ghafuri, H. Synlett 2009, 2245.
[24] Zhang, X.-X.; Li, W.-D. Chin. J. Org. Chem. 2003, 23, 1185 (in Chinese). (张兴贤, 李卫东, 有机化学, 2003, 23, 1185.)
[25] Zhang, X.-X.; Weng, G.-D.; Zhang, Y.-D.; Li, P.-C. Tetrahedron 2015, 71, 2595.
[26] Kashima, C.; Maruyama, T.; Harada, K.; Hibi, S.; Omote, Y. J. Chem. Res., Synop. 1988, 2, 62.
[27] Han, F.-G.; Lu, Y.; Ji, X.-M.; Zhao, M.-Q.; Zhang, X.-Y.; Liu, Y. Chin. J. Org. Chem. 2010, 30, 1080 (in Chinese). (韩富根, 卢叶, 姬小明, 赵铭钦, 张晓蕴, 刘云, 有机化学, 2010, 30, 1080.)
[28] Santus, R.; Patterson, L. K.; Giraud, M.; Valla, A.; Lafontant, P.; Bazin, M. Free Radical Biol. Med. 1994, 16, 801
/
| 〈 |
|
〉 |