

Chinese Journal of Organic Chemistry >
Synthesis and Properties of Porphyrin Containing Long Chain Alkylferrocene
Received date: 2015-09-06
Revised date: 2015-10-14
Online published: 2015-10-26
Supported by
Project supported by the National Natural Science Foundation of China (No. 21562032), the Research Program of Science and Technology at Universities of Inner Mongolia (No. NJZZ001) and the Natural Science Foundation of Inner Mongolia (Nos. 2013MS0207, 2014JQ02).
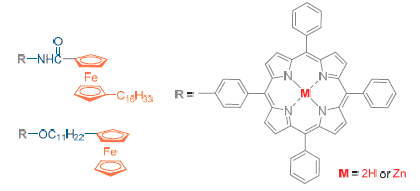
Two series of porphyrins and their Zn (II) complexes modified with long chain alkylferrocene were designed. One was close conjugate connection of porphyrin and ferrocene containing long chain alkyl group by an amide linkage in meso site of porphyrin (porphyrin 5 and its Zn (II) complex 6), the other was placed ferrocene on the skirt of meso-tetraphenylporphyrin with long chain alkoxy group (porphyrin 7 and its Zn(II) complex 8). The spectroscopic and electrochemistry properties of two series of compounds were investigated. The absorption bands of free porphyrins were almost the same, but those of their Zn(II) complexes were red-shifted by 7 nm. Porphyrins 5 and 6 with an amide linkage exhibited strong fluorescence quenching with a low quantum yield compared with those containing a long chain alkoxy group linkage (7 and 8). Compared with the free porphyrins, the negative potential shifts of the Zn(II) complexes were observed in cyclic voltammetry experiments. In addition, the first oxidation potentials of porphyrin in 7 and 8 were slight negative shifts relative to 5 and 6, but the first oxidation potentials of ferrocene in 7 and 8 shifted about 182~194 mV.
Chen Dong , Tuo Qiaoyan , Liu Wenxin , Yin Qin , Tong Xuguang , Zhao Haiying , Li Baoguo , Bian Zhanxi . Synthesis and Properties of Porphyrin Containing Long Chain Alkylferrocene[J]. Chinese Journal of Organic Chemistry, 2016 , 36(2) : 346 -351 . DOI: 10.6023/cjoc201509007
[1] Fungo, F.; Milanesio, M. E.; Durantini, E. N.; Otero, L.; Dittrich, T. J. Mater. Chem. 2007, 17, 2107.
[2] Vecchi, A.; Erickson, N. R.; Sabin, J. R.; Floris, B.; Conte, V.; Venanzi, M.; Galloni, P.; Nemykin, V. N. Chem. Eur. J. 2015, 21, 269.
[3] Fukuzumi, S.; Kojima, T. J. Mater. Chem. 2008, 18, 1427.
[4] Villegas, C.; Wolf, M.; Joly, D.; Delgado, J. L.; Guldi, D. M.; Martín, N. Org. Lett. 2015, 17(20), 5056.
[5] Rousseaux, S. A. L.; Gong, J. Q.; Haver, R.; Odell, B.; Claridge, T. D. W.; Herz, L. M.; Anderson, H. L. J. Am. Chem. Soc. 2015, 137, 12713.
[6] Tagliatesta, P.; Pizzoferrato, R. J. Organomet. Chem. 2015, 787, 27.
[7] Zhao, H.; Gu, X.; Yan, X.; Liu, Z.; Chen, Q.; Bian, Z. Chin. J. Org. Chem. 2014, 34, 371 (in Chinese). (赵海英, 顾雪松, 鄢小卿, 刘智波, 陈强, 边占喜, 有机化学, 2014, 34, 371.)
[8] Bucher, C.; Devillers, C. H.; Moutet, J.-C.; Saint-Aman, E. Coord. Chem. Rev. 2009, 253, 21.
[9] Gokulnath, S.; Achary, B. S.; Kumar C. K.; Trivedi, R.; Sridhar, B.; Giribabu, L. Photochem. Photobiol. 2015, 91, 33.
[10] Wijesinghe, C. A.; El-Khouly, M. E.; Fukuzumi, S.; D'Souza, F. Chem. Eur. J. 2013, 19, 9629.
[11] Lvova, L.; Galloni, P.; Floris, B.; Lundström, I.; Paolesse, R.; Natale, C. D. Sensors 2013, 13, 5841.
[12] Sirbu, D.; Turta, C.; Benniston, A. C.; Abou-Chahine, F.; Lemmetyinen, H.; Tkachenko, N. V.; Wood, C.; Gibson, E. RSC Adv. 2014, 4, 22733.
[13] Mittra, K; Chatterjee, S.; Samanta, S.; Dey, A. Inorg. Chem. 2013, 52, 14317.
[14] Sirbu, D.; Turta, C.; Gibson, E. A.; Benniston, A. C. Dalton Trans. 2015, 44, 14646.
[15] Vecchi, A.; Galloni, P.; Floris, B.; Nemykin, V. N. J. Porphyrins Phthalocyanines 2013, 17, 165.
[16] Brahma, S.; Ikbal, S. A.; Dhamija, A.; Rath, S. P. Inorg. Chem, 2014, 53, 2381.
[17] Sun, B.; Ou, Z.; Meng, D.; Fang, Y.; Song, Y.; Zhu, W.; Solntsev, P. V.; Nemykin, V. N.; Kadish, K. M. Inorg. Chem. 2014, 53, 8600.
[18] Zhu, P.; Kan, L.; Han, X.; Feng, J.; Jia, J.; Zhang, X. Dyes Pigm. 2015, 113, 55.
[19] Wang, L.; Li, B.; Shi, Y. Chin. J. Org. Chem. 2006, 26, 120 (in Chinese). (王丽英, 李保国, 石艳菊, 有机化学, 2006, 26, 120.)
[20] Majumdar, K. C.; Chakravorty, S.; Pal, N.; Sinha, R. K. Tetrahedron 2009, 65, 7998.
[21] Yu, L.; Wang, R.; Gao, B.; Geng, T.; Chen, M.; Lei, C.; Liu, S.; Huang, X.; Lei, M. Chin. J. Lumin. 2012, 33, 1373 (in Chinese). (喻龙, 王蕊欣, 高保娇, 耿天奇, 陈玫君, 雷彩萍, 刘少伟, 黄小利, 雷鸣, 发光学报, 2012, 33, 1373.)
/
| 〈 |
|
〉 |