

Chinese Journal of Organic Chemistry >
Density Functional Theory Study on Fluorine Abstraction of the CF3I Catalytic Synthesis
Received date: 2015-09-20
Revised date: 2015-10-17
Online published: 2015-10-26
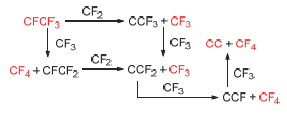
CF3I has been widely used in the fields such as refrigerants, etching agents, foaming agents, fire extinguishing agents and organic fluorine industry. By reacting CHF3 with I2 in the presence of a catalyst, CHF3 can transform into CF3I. Aiming at this catalytic process, possible reaction pathways for the generation of CF3 (an important intermediate), CF4 (a main byproduct) and coke (an important reason for the catalyst deactivation) are investigated with quantum chemistry methods using density functional theory (DFT). The results show that CF2 and CF3 could abstract the fluorine from CFCF3 in multisteps over graphite(001) surface to afford CF3, CF4 and C2 radical respectively. It is also found that the coke deposition in experiments is due to the above fluorine abstraction. The suggested mechanism is in agreement with available experimental data and theoretical computations.
Key words: CF3I; synthesis; CFCF3; fluorine abstraction; activated carbon
Hu Yingjie , Pan Renming , Wan Wanjun . Density Functional Theory Study on Fluorine Abstraction of the CF3I Catalytic Synthesis[J]. Chinese Journal of Organic Chemistry, 2015 , 35(12) : 2522 -2528 . DOI: 10.6023/cjoc201509022
[1] Levy, R. A.; Zaitsev, V. B.; Aryusook, K. J. Mater. Res. 1998, 13, 2643.
[2] Christophorou, L. G.; Olthoff, J. K. J. Phys. Chem. Ref. Data 2000, 29, 553.
[3] Li, Y.; Patten, K. O.; Youn, D. Atmos. Chem. Phys. 2006, 6, 4559.
[4] Hamins, A.; Borthwick, P. Combust. Flame 1998, 112, 161.
[5] Su, J. Z.; Kim, A. K.; Mawhinney, J. R. J. Fire Prot. Eng. 1996, 8, 45.
[6] Su, J. Z.; Kim A. K. Fire Technol. 2002, 38, 7.
[7] Vinegar, A.; Jepson, G. W.; Hammann, S. J. Am. Ind. Hyg. Assoc. J. 1999, 60, 403.
[8] Duan, Y. Y.; Zhu, M. S.; Shi, L. Fluid Phase Equilib. 1997, 131, 233.
[9] Petrik, V.; Cahard, D. Tetrahedron Lett. 2007, 48, 3327.
[10] Nagasaki, N.; Morikuni, Y.; Kawada, K.; Arai, S. Catal. Today 2004, 88, 121.
[11] Nagasaki, N.; Suzuki, N.; Nakano, S.; Kunihiro, N. US 5892136, 1999 [Chem. Abstr. 1998, 129, 29362].
[12] Yang, G. C.; Lei, S.; Pan, R. M.; Quan, H. D. J. Fluorine Chem. 2009, 130, 231.
[13] Yang, G. C.; Jia, X. Q.; Pan, R. M.; Quan, H. D. J. Mol. Catal. A: Chem. 2009, 309, 184.
[14] Yang, G. C.; Jia, X. Q.; Pan, R. M. J. Fluorine Chem. 2009, 130, 985.
[15] Hu, Y. J.; Wu, T. P.; Liu, W. Z.; Pan, R. M. J. Phys. Chem. A 2014, 118, 1918.
[16] Figueiredo, J.; Pereira, M.; Freitas, M.; Orfao, J. Carbon 1999, 37, 1379.
[17] McDougall, G. J. J. South. Afr. Inst. Min. Metall. 1991, 91, 109.
[18] Rodriguez-Reinoso, F. Carbon 1998, 36, 159.
[19] Delley, B. J. Chem. Phys. 2000, 113, 7756.
[20] Perdew, J. P.; Burke, K.; Ernzerhof, M. Phys. Rev. Lett. 1996, 77, 3865.
[21] Delley, B. J. Chem. Phys. 1990, 92, 508.
[22] Dolg, M.; Wedig, U.; Stoll, H.; Preuss, H. J. Chem. Phys. 1987, 86, 866.
[23] Bergner, A.; Dolg, M.; Küchle, W. Mol. Phys. 1993, 80, 1431.
[24] Halgren, T. A.; Lipscomb, W. N. Chem. Phys. Lett. 1977, 49, 225.
[25] Henkelman, G.; Jonsson, H. J. Chem. Phys. 2000, 113, 9978.
/
| 〈 |
|
〉 |