

Chinese Journal of Organic Chemistry >
Piperidinium Acetate-Catalyzed the Synthisis of 2,3-Disubstituted Chroman-4-one and 2,2-Disubstituted Benzofuran-3-one Derivatives
Received date: 2015-10-13
Revised date: 2015-11-03
Online published: 2015-11-16
Supported by
Project supported by the Natural Science Foundation of China (No. 21202042), the Hunan Provincial Natural Science Foundation of China (Nos. 13JJ4090, 2015JJ3063) and the Natural Science Foundation of Hunan University of Technology (No. 2014HZX01).
A piperidinium acetate-catalyzed method was developed for the Knoevenagel and O-Micheal cascade reaction of o-hydroxyphenyl-β-diketoes with different series of aldehydes. In the presence of piperidinium acetate, sixteen 2,3-disub-stituted chroman-4-ones and nine 2,2-disubstituted benzofuran-3-ones were obtained in moderate to good yields, and the cascade reaction displayed better substrate adaptability. Moreover, the reaction has the following features: a convenient and simple path, mild reaction conditions and a cheap catalyst.
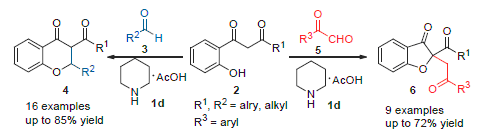
Key words: piperidinium acetate; cascade reaction; chroman-4-one; benzofuran-3-one
Wang Haifei , Zhou Zhipeng , Hu Shunqin , Wen Pushan , Tao Bin , Deng Qifu . Piperidinium Acetate-Catalyzed the Synthisis of 2,3-Disubstituted Chroman-4-one and 2,2-Disubstituted Benzofuran-3-one Derivatives[J]. Chinese Journal of Organic Chemistry, 2016 , 36(3) : 596 -603 . DOI: 10.6023/cjoc201510014
[1] (a) Akatsuka, T.; Kodama, O.; Kato, H. Agric. Boil. Chem. 1983, 47, 445.
(b) Kono, Y.; Takeuehi, S.; Kodama, O. Agric. Boil. Chem. 1984, 48, 253.
[2] (a) Hollingworth, R. M.; Ahammadsahib, K. I. Rev. Pestic. Toxicol. 1995, 3, 277.
(b) Zhou, Z. Z.; You, W. W. Chin. J. Org. Chem. 2008, 28, 1849 (in Chinese). (周中振, 游文玮, 有机化学, 2008, 28, 1849.)
[3] (a) Seikel, M. K.; Geissman, T. A. J. Am. Chem. Soc. 1950, 72, 5725.
(b) Nakayama, T.; Yonekura-Sakakibara, K.; Sato, T.; Kikuchi, S.; Fukui, Y.; Fukuchi-Mizutani, M.; Ueda, T.; Nakao, M.; Tanaka, Y. Science 2000, 290, 1163.
[4] (a) Pirrung, M. C.; Brown, W. L.; Rege, S.; Laughton, P. J. Am. Chem. Soc. 1991, 113, 8561.
(b) Nakajima, M.; Yamamoto, S.; Yamaguchi, Y.; Nakamura, S.; Hashimoto, S. Tetrahedron 2003, 59, 7307.
[5] (a) Hu, C.; Qiu, Z. Y.; Wang, Y. Y. Fine Chem. Intermed. 2010, 40, 19 (in Chinese). (胡春, 邱志远, 王永永, 精细化工中间体, 2010, 40, 19.)
(b) Zheng, W.; Sun, G.; Sun, B. J. Fine Chem. Intermed. 2009, 39, 30 (in Chinese). (郑伟, 孙光, 孙宝佳, 精细化工中间体, 2009, 39, 30.)
(c) Zheng, H.; Zou, Y.; Weng, L. L. Chin. J. Med. Chem. 1999, 9, 285 (in Chinese). (郑虎, 邹永, 翁玲玲, 中国药物化学杂志, 1999, 9, 285.)
(d) Wu, L. H.; Lin, Z. H.; Cai, L. J. Fine Chem. Intermed. 2010, 40, 38 (in Chinese). (吴利欢, 林真辉, 蔡立琼, 精细化工中间体, 2010, 40, 38.)
[6] (a) Su, X. Y.; Li, Y. L. J. Nat. Prod. 1996, 59, 968.
(b) Brown, M. K.; Degrado, S. J.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2005, 44, 5306.
(c) Biddle, M. M.; Lin, M.; Scheidt, K. A. J. Am. Chem. Soc. 2007, 129, 3830.
[7] (a) Nakib, A. T.; Bezjak, V.; Meegan, M. J. Eur. J. Med. Chem. 1990, 25, 455.
(b) Cheng, X. M.; Huang, Z. T.; Zheng, Q. Y. Tetrahedron 2011, 67, 9093.
[8] Wankhade, B. B.; Chincholkar, M. M. Asian J. Chem. 2000, 12, 1243.
[9] (a) McCarthy, N. M.; McKervey, A.; Ye, T.; McCann, M.; Murphy, E.; Doyle, M. P. Tetrahedron Lett. 1992, 33, 5983.
(b) Ferris, L.; Haigh, D.; Moody, C. J. Tetrahedron Lett. 1996, 37, 107.
(c) Ghosh, S.; Banerjee, I.; Baul, S. Tetrahedron 1999, 55, 11537.
(d) Hodgson, D. M.; Petroliagi, M. Tetrahedron: Asymmetry 2001, 12, 877.
[10] Filloux, C. M.; Lathrop, S. P.; Rovis, T. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 20666.
[11] (a) Fillion, E.; Fishlock, D. Org. Lett. 2003, 5, 4653.
(b) Zumbansen, K.; Döhring, A.; List, B. Adv. Synth. Catal. 2010, 352, 1135.
(c) Wang, H.; Luo, J.; Han, X.; Lu, Y. Adv. Synth. Catal. 2011, 353, 2971.
(d) Kim, M.; Jung, Y.; Kim, I. J. Org. Chem. 2013, 78, 10395.
/
| 〈 |
|
〉 |