

Chinese Journal of Organic Chemistry >
Application of Deep-Eutectic Solvents in Green Organic Synthesis
Received date: 2015-08-05
Revised date: 2015-10-31
Online published: 2015-12-04
Supported by
Project supported by the National Natural Science Foundation of China (No. 21004024), the Natural Science Foundation of Fujian Province (No. 2016J01063), the Program for New Century Excellent Talents in Fujian Province (No. 2012FJ-NCET-ZR03), the University Distinguished Young Research Talent Training Program of Fujian province (No. 11FJPY02) and the Promotion Program for Young and Middle-aged Teacher in Science and Technology Research of Huaqiao University (No. ZQN-YX103).
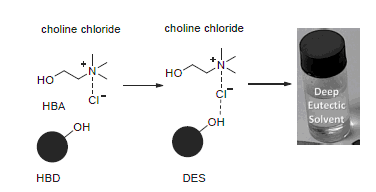
Since the definition of "green chemistry" was proposed in 1991, the green solvents have attracted considerable attention. In the two decades ago, ionic liquids grew up. However, as following the research, it was found that ionic liquids had some drawbacks. Nowadays, some new types of low-transition-temperature mixtures, i.e. deep-eutectic solvents (DES) are raised. Compared with the traditional ionic liquids, deep-eutectic solvents are greener, cheaper and more accessible. Furthermore, the preparation methods of deep-eutectic solvents are easy, and the components of them are biodegradable. In recent years, deep-eutectic solvents have been applied as green solvents in organic synthesis widely. In this review, the application of deep-eutectic solvents in cyclization reactions, replacement reactions, addition reactions, multicomponent reactions, enzyme catalytic reactions and other reactions in the last few years are briefly summarized, and the prospects of DESs are also discussed.
Key words: deep-eutectic solvents; green chemistry; organic synthesis; catalysis
Xiong Xingquan , Han Qian , Shi Lin , Xiao Shangyun , Bi Cheng . Application of Deep-Eutectic Solvents in Green Organic Synthesis[J]. Chinese Journal of Organic Chemistry, 2016 , 36(3) : 480 -489 . DOI: 10.6023/cjoc201508004
[1] Hallett, J. P.; Welton, T. Chem. Rev. 2011, 111, 3508.
[2] Walsh, D. A.; Lovelock, K. R. J.; Licence, P. Chem. Soc. Rev. 2010, 39, 418.
[3] Bideau, J. L.; Viau, L.; Vioux, A. Chem. Soc. Rev. 2011, 40, 907.
[4] Xiong, X. Q.; Yi, C.; Han, Q.; Shi, L. Chin. J. Catal. 2015, 36, 237.
[5] Plechkova, N. V.; Seddon, K. R. Chem. Soc. Rev. 2008, 37, 123.
[6] Zhang, Q. H.; Vigier, K. D. O.; Royer, S.; Jérôme, F. Chem. Soc. Rev. 2012, 41, 7108.
[7] Ruβ, C.; König, B. Green Chem. 2012, 14, 2969.
[8] Smith, E. L.; Abbott, A. P.; Ryder, K. S. Chem. Rev. 2014, 114, 11060.
[9] Abboott, A. P.; Capper, G.; Davies, D. L.; Rasheed, R. K.; Tambyrajah, V. Chem. Commun. 2003, 70.
[10] Imperato, G.; Höger, S.; Lenoir, D.; König, B. Green Chem. 2006, 8, 1051.
[11] Imperato, G.; Eibler, E.; Niedermaier, J.; König, B. Chem. Commun. 2005, 1170.
[12] Ilgen, F.; König, B. Green Chem. 2009, 11, 848.
[13] Singh, B. S.; Lobo, H. R.; Pinjari, D. V.; Jarag, K. J.; Pandit, A. B.; Shankarling, G. S. Ultrason. Sonochem. 2013, 20, 633.
[14] Zhang, Z.-H.; Zhang, X.-N.; Mo, L.-P.; Li, Y.-X.; Ma, F.-P. Green Chem. 2012, 14, 1502.
[15] Chen, Z.; Zhou, B.; Cai, H.; Zou, X. Green Chem. 2009, 11, 275.
[16] Phadtare, S. B.; Shankarling, G. S. Green Chem. 2010, 12, 458.
[17] Singh,B.; Lobo, H.; Shankarling, G. Catal. Lett. 2011, 141, 178.
[18] De Santi, V.; Cardellini, F.; Brinchi, L.; Germani, R. Tetrahedron Lett. 2012, 53, 5151.
[19] Imperato, G.; Vasold, R.; König, B. Adv. Synth. Catal. 2006, 348, 2243.
[20] Wang, A. L.; Xing, P. F.; Zheng, X. L.; Cao, H. Y.; Yang, G.; Zheng, X. F. RSC Adv. 2015, 5, 59022.
[21] Pawar, P. M.; Jarag, K. J.; Shankarling, G. S. Green Chem. 2011, 13, 2130.
[22] Singh, B. S.; Lobo, H. R.; Shankarling, G. S. Catal. Commun. 2012, 2470.
[23] Sonawane, Y. A.; Phadtare, S. B.; Borse, B. N.; Jagtap, A. R.; Shankarling, G. S. Org. Lett. 2010, 12, 1456.
[24] Azizi, N.; Gholibeglo, E. RSC Adv. 2012, 2, 7413.
[25] Azizi, N.; Rahimi, Z.; Alipour, M. RSC Adv. 2015, 5, 61191.
[26] Iha, R. K.; Wooley, K. L.; Nystrom, A. M.; Burke, D. J.; Kade, M. J.; Hawker, C. J. Chem. Rev. 2009, 109, 5620.
[27] Azizi, N.; Mariami, M.; Edrisi, M. Dyes Pigm. 2014, 100, 215.
[28] Keshavarzipour, F.; Tavakol, H. Catal. Lett. 2015, 145, 1062.
[29] Gorke, J. T.; Srienc F.; Kazlauskas, R. J. Chem. Commun. 2008, 1235.
[30] Zhao, H.; Baker, G. A.; Holmes, S. Org. Biomol. Chem. 2011, 9, 1908.
[31] Zhao, H. Zhang, C.; Crittle, T. D. J.Mol. Catal. B: Enzym. 2013, 85.
[32] Azizi, N.; Batebi, E.; Bagherpour, S.; Ghafuri, H. RSC Adv. 2012, 2, 2289.
[33] Coulembier, O.; Lemaur, V.; Josse, T.; Minoia, A.; Cornil, J.; Dubois, P. Chem. Sci. 2012, 3, 723.
[34] Ilgen, F.; Ott, D.; Kralisch, D.; Reil, C.; Palmbergera, A.; König, B. Green Chem. 2009, 11, 1948.
[35] Hu, S.; Zhang, Z.; Zhou, Y.; Han, B.; Fan, H.; Li, W.; Song, J.; Xie, Y. Green Chem. 2008, 10, 1280.
[36] Hu, S.; Zhang, Z.; Zhou, Y.; Song, J.; Fan, H.; Han, B. Green Chem. 2009, 11, 873.
[37] De Oliveira Vigier, K.; Benguerba, A.; Barrault, J.; Jérôme, F. Green Chem. 2012, 14, 285.
[38] Patil, U. B.; Singh, A. S.; Nagarkar, J. M. RSC Adv. 2014, 4, 1102.
/
| 〈 |
|
〉 |