

Chinese Journal of Organic Chemistry >
Synthesis and Protein-Tyrosine Phosphatase 1B Inhibitory Activity Evaluation of Indolepropyl Based 1,3,4-Oxadiazole Derivatives
Received date: 2015-09-21
Revised date: 2015-11-12
Online published: 2015-12-04
Supported by
Project supported by the National Marine "863" Projects (Nos. 2013AA092902 and 2012AA092105), the National Natural Science Foundation of China (Nos. 81520108028, 81273430, 41306130, 41506187, 81302692, 41476063), the NSFC-Shandong Joint Fund for Marine Science Research Centers (No. U1406402), the Science and Technology Commission of Shanghai Municipality Project (Nos. 14431901100, 15431901000), the State Key Laboratory of Drug Research/Shanghai Institute of Materia Medica Projects (Nos. SIMM1501ZZ-03, CASIMM0120152039).
A series of indolepropyl based 1,3,4-oxadiazole derivatives were synthesized by esterification, hydrazidation, cyclization and substitution using indolebutyric acid as starting material. The inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was evaluated. The results indicated that five compounds displayed obviously inhibitory effect against PTP1B in vitro, for instance, compound 5g exhibited the strongest PTP1B inhibitory activity with an IC50 value of 6.74 μg· mL-1 . It was the first example of indolealkyl based oxadiazole derivatives showing PTP1B inhibitory activity.
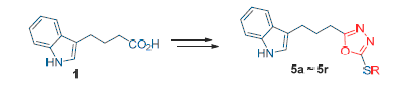
Key words: 1,3,4-oxadiazole; synthesis; PTP1B inhibitory activity
Mei Wenwen , Guo Yuewei , Li Jia , Cai Meiyi , Ma Wenquan , Gong Jingxu , Wang Xuedong . Synthesis and Protein-Tyrosine Phosphatase 1B Inhibitory Activity Evaluation of Indolepropyl Based 1,3,4-Oxadiazole Derivatives[J]. Chinese Journal of Organic Chemistry, 2016 , 36(3) : 533 -539 . DOI: 10.6023/cjoc201509024
[1] Jiang, C.-S.; Liang, L.-F.; Guo, Y.-W. Acta Pharmacol. Sin. 2012, 33, 1217.
[2] Safavi, M.; Foroumadi, A.; Abdollahi, M. Expert Opin. Drug Discovery 2013, 8, 1339.
[3] Miller, B. R.; Nguyen, H.; Hu, C. J.; Lin, C.; Nguyen, Q. T. Am Health Drug Benefits 2014, 7, 452.
[4] Tamrakar, A. K.; Maurya, C. K.; Rai, A. K. Expert Opin. Ther. Pat. 2014, 24, 1101.
[5] Feldhammer, M.; Uetani, N.; Miranda-Saavedra, D.; Tremblay, M. L. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 430.
[6] Li, Y.-J.; Shao, X.; Gao, L. X.; Jin, K.; Sheng, L.; Luo, T.-C.; Yu, Y.; Li, J. Chin. J. Org. Chem. 2013, 33, 2178 (in Chinese). (李英俊, 邵昕, 高立信, 靳焜, 盛丽, 罗潼川, 于洋, 李佳, 有机化学, 2013, 33, 2178.)
[7] Li, Y.-F.; Liang, L.-F.; Xiao, W.; Liang, J.-Y.; Guo, Y.-W. Chin. J. Org. Chem. 2013, 33, 1157 (in Chinese). (李玉芬, 梁林富, 萧伟, 梁敬钰, 郭跃伟, 有机化学, 2013, 33, 1157.)
[8] Zhao, C.-Y.; Zhu, T.-H.; Zhu, W.-M. Chin. J. Org. Chem. 2013, 33, 1195 (in Chinese). (赵成英, 朱统汉, 朱伟明, 有机化学, 2013, 33, 1195.)
[9] Chen, W.; Hao, H.-l.; Zhang, C.-l.; Shen, Y.-M. Chin. J. Org. Chem. 2014, 34, 797 (in Chinese). (陈旺, 郝慧琳, 张晨露, 沈月毛, 有机化学, 2014, 34, 797.)
[10] Zhou, N.-N.; Shi, Q.; Xie, Z.-X. Chin. J. Org. Chem. 2014, 34, 1104 (in Chinese). (周妮妮, 史倩, 谢志翔, 有机化学, 2014, 34, 1104.)
[11] Wang, S.; Luan, H.; Wu, C.-M.; Guo, P. Int. J. Pharm. Res. 2014, 41, 537 (in Chinese). (王帅, 栾 虹, 吴崇明, 郭鹏, 国际药学研究杂志. 2014, 41, 537.)
[12] Xue, D.-Q.; Wang, J.-J.; Liu, H.-L.; Guo, Y.-W. Chin. J. Nat. Med. 2009, 7, 150 (in Chinese). (薛多清, 王俊杰, 刘海利, 郭跃伟, 中国天然药物, 2009, 7, 150.)
[13] Carbone, M.; Li, Y.; Irace, C.; Mollo, E.; Castelluccio, F.; Pascale, A. D.; Cimino, G.; Santamaria, R.; Guo, Y.-W.; Gavagnin, M. Org. Lett. 2011, 13, 2516.
[14] Lin, H. Y.; Snider, B. B. J. Org. Chem. 2012, 77, 4832.
[15] Brogan, J. T.; Stoops, S. L.; Lindsley, C. W. ACS Chem. Neurosci. 2012, 3, 658.
[16] Manzo, E.; Pagano, D.; Carbone, M.; Ciavatta, M. L.; Gavagnin, M. ARKIVOC (Gainesville, FL, U. S.) 2012, 9, 220.
[17] Buchanan, J. C.; Petersen, B. P.; Chamberland, S. Tetrahedron Lett. 2013, 54, 6002.
[18] Vitale, R. M.; Gatti, M.; Carbone, M.; Barbieri, F.; Felicita, V.; Gavagnin, M.; Florio, T.; Amodeo, P. ACS Chem. Biol. 2013, 8, 2762.
[19] Jiang, C.-S.; Fu, Y.; Zhang, L.; Gong, J.-X.; Wang, Z.-Z.; Xiao, W.; Zhang, H.-Y.; Guo, Y.-W. Bioorg. Med. Chem. Lett. 2015, 25, 216.
[20] Athare, C. L.; Upasani, C. D. Int. J. Pharm. Phytopharm. Res. 2014, 3, 383.
[21] Chen, Z.-H.; Sun, L.-P.; Zhang, W.; Shen, Q.; Gao, L.-X.; Li,-J.; Piao, H.-R. Bull. Korean Chem. Soc. 2012, 33, 1505.
[22] Rakse, M.; Karthikeyan, C.; Deora, G. S.; Moorthy, N. S.; Rathore, V.; Rawat, A. K.; Srivastava, A. K.; Trivedi, P. Eur. J. Med. Chem. 2013, 70, 469.
[23] He, Y.-W.; Cao, L.-H.; Zhang, J.-B.; Wang, D.-Z.; Aisa, H. A. Carbohydr. Res. 2011, 346, 551.
[24] Hansen, S. K.; Cancilla, M. T.; Shiau, T. P.; Kung, J.; Chen, T.; Erlanson, D. A. Biochemistry 2005, 44, 7704.
[25] Dabiri, M.; Salehi, P.; Baghbanzadeha, M.; Bahramnejad, M. Tetrahedron Lett. 2006, 47, 6983.
[26] Zhang, K.; Wang, P.; Xuan, L.-N.; Fu, X.-Y.; Jing, F.; Li, S.; Liu, Y.-M.; Chen, B.-Q. Bioorg. Med. Chem. Lett. 2014, 54, 5154.
[27] El-Din, M. M. G.; El-Gamal, M. I.; Abdel-Maksoud, M. S.; Yoo, K. H.; Oh, C. H. Eur. J. Med. Chem. 2015, 90, 45.
[28] Zhang, M. Z.; Mulholland, N.; Beattie, D.; Irwin, D.; Gu, Y. C.; Chen, Q.; Yang, G. F.; Clough, J. Eur. J. Med. Chem. 2013, 63, 22.
[29] Li, P.; Shi, L.; Gao, M.-N.; Yang, X.; Xue, W.; Jin, L. H.; Hu, D.-Y.; Song, B.-A. Bioorg. Med. Chem. Lett. 2015, 25, 481.
[30] Misra, U.; Hitkari, A.; Saxena, A. K.; Gurtu, S.; Shanker, K. Eur. J. Med. Chem. 1996, 31, 629.
[31] de la Fuente Revenga, M.; Fernandez-Saez, N.; Herrera-Arozamena, C.; Morales-García, J. A.; Alonso-Gil, S.; Pérez-Castillo, A.; Caignard, D. H.; Rivara, S.; Rodríguez-Franco, M. I. J. Med. Chem. 2015, 58, 4998.
[32] Chu, Y.; Lu, D.; Xu, M.-X. Chin. J. Med. Chem. 2001, 39, 37 (in Chinese). (楚勇, 吕丁, 徐鸣夏, 中国药物化学杂志, 2001, 39, 37.)
[33] Rapolu, S.; Alla, M.; Bommena, V. R.; Murthy, R.; Jain, N.; Bommareddy, V. R.; Bommineni, M. R. Eur. J. Med. Chem. 2013, 66, 81.
/
| 〈 |
|
〉 |