

Chinese Journal of Organic Chemistry >
Application of Sulfonyl in Drug Design
Received date: 2015-10-09
Revised date: 2015-11-13
Online published: 2015-12-04
Supported by
Project supported by the National Natural Science Foundation of China (No. 21372235).
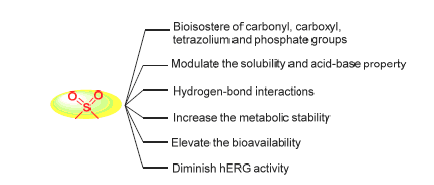
Sulfonyl-containing compounds comprise a substantial proportion in the therapeutic drugs. It is an important strategy to introduce sulfonyl group into the small molecules for structure-based medicinal chemistry, the application of sulfonyl group in drug design is reviewed in this paper. Structurally, sulfonyl group has similar properties in molecular size and charge distribution with carbonyl, carboxyl, tetrazolium and phosphate group, so it can be introduced into the drug molecules as their bioisostere in order to remain or improve activity. Sulfonyl group possesses unique physicochemical properties, and the introduction of sulfonyl group can also modulate the solubility and acid-base property of the drug molecules. Sulfonyl group can offer two hydrogen-bond receptors, and reasonable introduction of sulfonyl group can enhance the binding affinity of the drug molecules with the targeted proteins to improve activity through hydrogen bond interactions. What's more, sulfonyl group is relatively stable in terms of structure, the introduction of sulfonyl group can increase the metabolic stability of drugs to prolong the duration of action by blocking metabolically labile sites, improve the pharmacokinetic properties of the drug molecules to elevate the bioavailability. Sulfonyl group belongs to polar groups, which can also increase the polarity of the drug molecules to diminish hERG activity.
Key words: sulfonyl; drug design; lead optimization
Zhao Fei , Wang Jiang , Ding Xiao , Shu Shuangjie , Liu Hong . Application of Sulfonyl in Drug Design[J]. Chinese Journal of Organic Chemistry, 2016 , 36(3) : 490 -501 . DOI: 10.6023/cjoc201510006
[1] Penning, T. D.; Talley, J. J.; Bertenshaw, S. R.; Carter, J. S.; Collins, P. W.; Docter, S.; Graneto, M. J.; Lee, L. F.; Malecha, J. W.; Miyashiro, J. W.; Rogers, R. S.; Rogier, D. J.; Yu, S. S.; Anderson, G. D.; Burton, E. G.; Cogburn, J. N.; Gregory, S. A.; Koboldt, C. M.; Perkins, W. E.; Seibert, K.; Veenhuizen, A. W.; Zhang, Y. Y.; Isakson, P. C. J. Med. Chem. 1997, 40, 1347.
[2] Takenaka, T.; Honda, K.; Fujikura, T. J. Pharm. Pharmacol. 1984, 36, 539.
[3] Terrett, N. K.; Bell, A. S.; Brown, D.; Ellis, P. Bioorg. Med. Chem. Lett. 1996, 6, 1819.
[4] Cross, P. E.; Arrowsmith, J. E.; Thomas, G. N.; Gwilt, M.; Burges, R. A.; Higgins, A. J. J. Med. Chem. 1990, 33, 1151.
[5] Quesnel, L. B.; Al-Najjar, A. R.; Buddhavudhikrai, P. J. Appl. Bacteriol. 1978, 45, 397.
[6] Uchida, T.; Hayashi, K.; Kido, H.; Watanabe, M. J. Pharm. Pharmacol. 1992, 44, 39.
[7] Grell, W.; Hurnaus, R.; Griss, G.; Sauter, R.; Rupprecht, E.; Mark, M.; Luger, P.; Nar, H.; Wittneben, H.; Müller, P. J. Med. Chem. 1998, 41, 5219.
[8] Baguley, B. C.; Denny, W. A.; Atwell, G. J.; Cain, B. F. J. Med. Chem. 1981, 24, 520.
[9] Colas, B.; Slama, M.; Masson, H.; Colas, J. L.; Collin, T.; Arnould, M. L.; Hary, L.; Safar, M.; Andrejak, M. Fundam. Clin. Pharmacol. 2000, 14, 363.
[10] Sharghi, N.; Lalezari, N. S.; Niloofari, G.; Golgolab, H. J. Med. Chem. 1969, 12, 696.
[11] Miyashita, M.; Matsumoto, T.; Matsukubo, H.; Iinuma, F.; Taga, F.; Sekiguchi, H.; Hamada, K.; Okamura, K.; Nishino, K. J. Med. Chem. 1992, 35, 2446.
[12] Högberg, T.; Bengtsson, S.; Depaulis, T.; Johansson, L.; Ström, P.; Hall, H.; Ogren, S. O. J. Med. Chem. 1990, 33, 1155.
[13] Roushdi, I. M.; Mikhail, A. A.; Chaaban, I. Pharmazie 1977, 32, 269.
[14] Leston, S.; Nebot, C.; Nunes, M.; Cepeda, A.; Pardal, M. A.; Ramos, F. Environ. Toxicol. Pharmacol. 2015, 39, 77.
[15] Vandyck, K.; Cummings, M. D.; Nyanguile, O.; Boutton, C. W.; Vendeville, S.; Mcgowan, D.; Devogelaere, B.; Amssoms, K.; Last, S.; Rombauts, K.; Tahri, A.; Lory, P.; Hu, L.; Beauchamp, D. A.; Simmen, K.; Raboisson, P. J. Med. Chem. 2009, 52, 4099.
[16] Hunt, J. T.; Ding, C. Z.; Batorsky, R.; Bednarz, M.; Bhide, R.; Cho, Y.; Chong, S.; Chao, S.; Gullo-Brown, J.; Guo, P.; Kim, S. H.; Lee, F. Y.; Leftheris, K.; Miller, A.; Mitt, T.; Patel, M.; Penhallow, B. A.; Ricca, C.; Rose, W. C.; Schmidt, R.; Slusarchyk, W. A.; Vite, G.; Manne, V. J. Med. Chem. 2000, 43, 3587.
[17] Kallander, L. S.; Washburn, D. G.; Hoang, T. H.; Frazee, J. S.; Stoy, P.; Johnson, L.; Lu, Q.; Hammond, M.; Barton, L. S.; Patterson, J. R.; Azzarano, L. M.; Nagilla, R.; Madauss, K. P.; Williams, S. P.; Stewart, E. L.; Duraiswami, C.; Grygielko, E. T.; Xu, X.; Laping, N. J.; Bray, J. D.; Thompson, S. K. Bioorg. Med. Chem. Lett. 2010, 20, 371.
[18] Cabrera, D. G.; Douelle, F.; Younis, Y.; Feng, T. S.; Manach, C. L.; Nchinda, A. T.; Street, L. J.; Scheurer, C.; Kamber, J.; White, K. L.; Montagnat, O. D.; Ryan, E.; Katneni, K.; Zabiulla, K. M.; Joseph, J. T.; Bashyam, S.; Waterson, D.; Witty, M. J.; Charman, S. A.; Wittlin, S.; Chibale, K. J. Med. Chem. 2012, 55, 11022.
[19] Bahl, A.; Barton, P.; Bowers, K.; Brough, S.; Evans, R.; Luckhurst, C. A.; Mochel, T.; Perry, M. W.; Rigby, A.; Riley, R. J.; Sanganee, H.; Sisson, A.; Springthorpe, B. Bioorg. Med. Chem. Lett. 2012, 22, 6688.
[20] Alexiou, P.; Demopoulos, V. J. J. Med. Chem. 2010, 53, 7756.
[21] Combs, A. P.; Yue, E. W.; Bower, M.; Ala, P. J.; Wayland, B.; Douty, B.; Takvorian, A.; Polam, P.; Wasserman, Z.; Zhu, W.; Crawley, M. L.; Pruitt, J.; Sparks, R.; Glass, B.; Modi, D.; McLaughlin, E.; Bostrom, L.; Li, M.; Galya, L.; Blom, K.; Hillman, M.; Gonneville, L.; Reid, B. G.; Wei, M.; Becker-Pasha, M.; Klabe, R.; Huber, R.; Li, Y.; Hollis, G.; Burn, T. C.; Wynn, R.; Liu, P.; Metcalf, B. J. Med. Chem. 2005, 48, 6544.
[22] Roda, A.; Cerrè, C.; Manetta, A. C.; Cainelli, G.; Umani-Ronchi, A.; Panunzio, M. J. Med. Chem. 1996, 39, 2270.
[23] Breslin, H. J.; Lane, B. M.; Ott, G. R.; Ghose, A. K.; Angeles, T. S.; Albom, M. S.; Cheng, M.; Wan, W.; Haltiwanger, R. C.; Wells-Knecht, K. J.; Dorsey, B. D. J. Med. Chem. 2012, 55, 449.
[24] Kamisuki, S.; Shirakawa, T.; Kugimiya, A.; Abu-Elheiga, L.; Choo, H. Y.; Yamada, K.; Shimogawa, H.; Wakil, S. J.; Uesugi, M. J. Med. Chem. 2011, 54, 4923.
[25] Dangelo, N. D.; Kim, T. S.; Andrews, K.; Booker, S. K.; Caenepeel, S.; Chen, K.; Damico, D.; Freeman, D.; Jiang, J.; Liu, L.; McCarter, J. D.; Miguel, T. S.; Mullady, E. L.; Schrag, M.; Subramanian, R.; Tang, J.; Wahl, R. C.; Wang, L.; Whittington, D. A.; Wu, T.; Xi, N.; Xu, Y.; Yakowec, P.; Yang, K.; Zalameda, L. P.; Zhang, N.; Hughes, P.; Norman, M. H. J. Med. Chem. 2011, 54, 1789.
[26] Asada, M.; Obitsu, T.; Kinoshita, A.; Nakai, Y.; Nagase, T.; Sugimoto, I.; Tanaka, M.; Takizawa, H.; Yoshikawa, K.; Sato, K.; Narita, M.; Ohuchida, S.; Nakai, H.; Toda, M. Bioorg. Med. Chem. Lett. 2010, 20, 2639.
[27] Scola, P. M.; Sun, L.-Q.; Chen, J.; Wang, A. X.; Sit, S.-Y.; Chen, Y.; D'Andrea, S. V.; Zheng, Z.; Sin, N.; Venables, B. L.; Cocuzza, A.; Bilder, D.; Carini, D.; Johnson, B.; Good, A. C.; Rajamani, R.; Klei, H. E.; Friborg, J.; Barry, D.; Levine, S.; Chen, C.; Sheaffer, A.; Hernandez, D.; Falk, P.; Yu, F.; Zhai, G.; Knipe, J. O.; Mosure, K.; Shu, Y.-Z.; Phillip, T.; Arora, V. K.; Loy, J.; Adams, S.; Schartman, R.; Browning, M.; Levesque, P. C.; Li, D.; Zhu, J. L.; Sun, H.; Pilcher, G.; Bounous, D.; Lange, R. W.; Pasquinelli, C.; Eley, T.; Colonno, R.; Meanwell, N. A.; McPhee. F. Presented at the 239th National Meeting and Exposition of the American Chemical Society, San Francisco, CA, March 21~25, 2010; MEDI-38.
[28] Chakravarty, P. K.; Naylor, E. M.; Chen, A.; Chang, R. S.; Chen, T. B.; Faust, K. A.; Lotti, V. J.; Kivlighn, S. D.; Gable, R. A.; Zingaro, G. J. J. Med. Chem. 1994, 37, 4068.
[29] Pinard, E.; Alberati, D.; Borroni, E.; Fischer, H.; Hainzl, D.; Jolidon, S.; Moreau, J. L.; Narquizian, R.; Nettekoven, M.; Norcross, R. D.; Stalder, H.; Thomas, A. W. Bioorg. Med. Chem. Lett. 2008, 18, 5134.
[30] Sturino, C. F.; O'Neill, G.; Lachance, N.; Boyd, M.; Berthelette, C.; Labelle, M.; Li, L.; Roy, B.; Scheigetz, J.; Tsou, N.; Aubin, Y.; Bateman, K. P.; Chauret, N.; Day, S. H.; Lévesque, J. F.; Seto, C.; Silva, J. H.; Trimble, L. A.; Carriere, M. C.; Denis, D.; Greig, G.; Kargman, S.; Lamontagne, S.; Mathieu, M. C.; Sawyer, N.; Slipetz, D.; Abraham, W. M.; Jones, T.; McAuliffe, M.; Piechuta, H.; Nicoll-Griffith, D. A.; Wang, Z.; Zamboni, R.; Young, R. N.; Metters, K. M. J. Med. Chem. 2007, 50, 794.
[31] Jamieson, C.; Moir, E. M.; Rankovic, Z.; Wishart, G. J. Med. Chem. 2006, 49, 5029.
[32] Kazmierski, W. M.; Anderson, D. L.; Aquino, C.; Chauder, B. A.; Duan, M.; Ferris, R.; Kenakin, T.; Koble, C. S.; Lang, D. G.; McIntyre, M. S.; Peckham, J.; Watson, C.; Wheelan, P.; Spaltenstein, A.; Wire, M. B.; Svolto, A.; Youngman, M. J. Med. Chem. 2011, 54, 3756.
[33] Shu, M.; Loebach, J. L.; Parker, K. A.; Mills, S. G.; Chapman, K. T.; Shen, D. M.; Malkowitz, L.; Springer, M. S.; Gould, S. L.; DeMartino, J. A.; Siciliano, S. J.; Salvo, J. D.; Lyons, K.; Pivnichny, J. V.; Kwei, G. Y.; Carella, A.; Carver, G.; Holmes, K.; Schleif, W. A.; Danzeisen, R.; Hazuda, D.; Kessler, J.; Lineberger, J.; Miller, M. D.; Emini, E. A. Bioorg. Med. Chem. Lett. 2004, 14, 947.
/
| 〈 |
|
〉 |