

Chinese Journal of Organic Chemistry >
Synthesis, Structure Characterization and Fungicidal Activity of 1,3-Disubstituted-1H-1,2,4-triazole-5-amines
Received date: 2015-10-19
Revised date: 2015-11-19
Online published: 2015-12-10
Supported by
Project supported by the National Natural Science Foundation of China (No. 31272076) and the National Scientific and Technology Supporting Program of China (No. 2011BAE06B05-5).
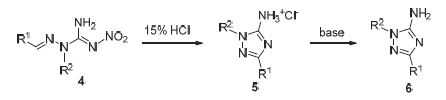
A novel series of 1,3-disubstituted-1H-1,2,4-triazole-5-amines were synthesized via hydrazinolysis, condensation, alkylation and acid-catalyzed intramolecular cyclization starting from nitroguanidine 1. Their structures were characterized by IR, 1H NMR, 13C NMR and HRMS analysis. The preliminary bioassay indicated that most of them showed certain extent of growth inhibition in vitro against six phytopathogen fungi. Compared with other target compounds, 1-(n-butyl)-3- (4-chlorophenyl)-1H-1,2,4-triazole-5-amine (6y) was found to have high activity and the widest fungicidal spectrum. Its inhibition rates to five pathogens were all above 53%. This kind of compounds could be synthesized and derived easily, and were valuable as lead structure for future developing novel fungicides.
Jia Changqing , Su Wangcang , Xu Yanjun , Liu Jiping , Qin Zhaohai . Synthesis, Structure Characterization and Fungicidal Activity of 1,3-Disubstituted-1H-1,2,4-triazole-5-amines[J]. Chinese Journal of Organic Chemistry, 2016 , 36(4) : 830 -837 . DOI: 10.6023/cjoc201510021
[1] Barbuceanu, S. F.; saramet, G.; Almajan, G. L.; Draghici, C.; Barbuceanu. F.; Bancescu, G. Eur. J. Med. Chem. 2012, 49, 417.
[2] Sato, T.; Ashizawa, N.; Iwanaga, T.; nakamura, H.; Matsumoto, K.; Inoue, T.; Nagata, O. Bioorg. Med.Chem. Lett. 2009, 19, 184.
[3] Papakonstantinou-Garoufalias, S.; Pouli, N.; marakos, P.; Chytyroglou-Ladas, A. Farmaco 2002, 57, 973.
[4] Abdel-Megeed, A. M.; Abdel-Rahman, H. M.; Alkaramany, G. S.; EI-Gendy, M. A. Eur. J. Med. Chem. 2009, 44, 117.
[5] Mallikarjuna, B. P.; Sastry, B. S.; Suresh Kumar, G. V.; Rajendraprasad, Y.; Chandrashekar, S. M.; Sathisha, K. Eur. J. Med. Chem. 2009, 44, 4739.
[6] Mange, Y. J.; Isloor, A. M.; Malladi, S.; Isloor, S. Arabian J. Chem. 2011, 6, 177.
[7] Zhang, R. B.; Lu, J. R.; Xin, C. W.; Liu, J. B.; Mu, J. B.; Yang, X. Y.; Wang, H. Y.; Wang, M. J.; Zhang, H. Chin. J. Org. Chem. 2015, 35, 858 (in Chinese). (张瑞波, 卢俊瑞, 辛春伟, 刘金彪, 穆江蓓, 杨旭云, 王宏韫, 王美君, 张贺, 有机化学, 2015, 35, 858.)
[8] Yu, G. P.; Xu, L. Z.; Yi, X.; Bi, W. Z.; Zhu, Q.; Zhai, Z. Z. J. Agric. Food Chem. 2009, 57, 4854.
[9] Arnoldi, A.; Dallavalle, S.; Merlini, L.; Musso, L.; Farina, G.; Moretti, M.; Jayasinghe, L. J. Agric. Food Chem. 2007, 55,8187.
[10] Ma, Y. M.; Liu, R. H.; Gong, X. Y.; Li, Z.; Huang, Q. C.; Wang, H. S.; Song, G. H. J. Agric. Food Chem. 2006, 54, 7724.
[11] Fan, Z. J.; Yang, Z. K.; Zhang, H. K.; Mi, N.; Wang, H.; Cai, F.; Zuo, X.; Zheng, Q. X.; Song, H. B. J. Agric. Food Chem. 2010, 58, 2630.
[12] Wang, H. Q.; Zhou, W. P.; Wang, Y. Y.; Lin, C. R. J. Agric. Food Chem. 2008, 56, 7321.
[13] Huang, W.; Zhao, P. L.; Liu, C. L.; Chen, Q.; Liu, Z. M.; Yang, G. F. J. Agric. Food Chem. 2007, 55, 3004.
[14] Cao, X.; Li, F.; Hu, M.; Lu, W.; Yu, G. A.; Liu, S. H. J. Agric. Food Chem. 2008, 56, 11367.
[15] Chen, X.; Yang, C. L. J. Agric. Food Chem. 2009, 57, 2441.
[16] Wang, B. L.; Shi, Y. X.; Ma, Y.; Liu, X. H.; Li, Y. H.; Song, H. B.; Li, B. J.; Li, Z. M. J. Agric. Food Chem. 2010, 58, 5515.
[17] Zhang, C. L.; Wang, X.; Guo, Y.; Wu, Y. F.; Gao, L. N.; Sun, L. J.; Chai, J. H.; Zhu, C. A. Chin. J. Org. Chem. 2014, 34, 2331 (in Chinese). (张成路, 王雪, 国阳, 吴一非, 高丽娜, 孙丽杰, 柴金华, 朱长安, 有机化学, 2014, 34, 2331.)
[18] Wang, B. L.; Shi, Y. X.; Zhan, Y. Z.; Zhang, L. Y.; Zhang, Y.; Wang, L. Z.; Zhang, X.; Li, Y. H.; Li, Z. M.; Li, B. J. Chin. J. Chem. 2015, 33, 1124.
[19] Su, W. C.; Zhou, Y. H.; Ma, Y. Q.; Wang, L.; Zhang, Z.; Rui, C. H.; Duan, H. X.; Qin, Z. H. J. Agric. Food Chem. 2012, 60, 5028.
[20] Yan, D. Y.; Wang, L.; Jia, C. Q.; Li, C. S.; Ma, Y. Q.; Rui, C. H.; Xu, Y. J.; Qin, Z. H. Chem. J. Chin. Univ. 2014, 35, 1703 (in Chinese).
/
| 〈 |
|
〉 |