

Chinese Journal of Organic Chemistry >
Synthesis and Antibacterial Activities of Novel Quinazoline-2,4-dione Derivatives Containing the 1,2,4-Triazole Schiff-Base Unit
Received date: 2015-10-09
Revised date: 2015-11-05
Online published: 2015-12-10
Supported by
Project supported by the National Natural Science Foundation of China (No. 21362003).
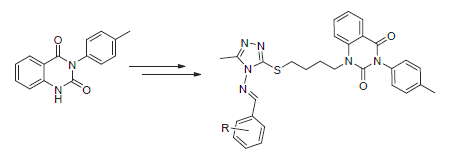
Eighteen novel quinazoline-2,4-dione derivatives containing the 1,2,4-triazole Schiff-base unit were designed and synthesized, based on the connection method of active fragments. Their structures were characterized by 1H NMR, 13C NMR, MS, IR and elemental analysis. The preliminary antibacterial test indicated that all the target compounds possessed an excellent inhibition activity (≥ 93%) against Xanthomonas oryzae pv. oryzae (Xoo) at the concentration of 200 μg/mL, which was obviously better than those of control agents thiodiazole-copper and bismerthiazol. Compounds 7a, 7c~7g and 7j~7l still displayed 100% inhibition rate against Xoo even at the concentration of 100 μg/mL. Moreover, all the target compounds exhibited a certain inhibition activity against Xanthomonas axonopodis pv. citri, but no inhibition activity against Ralstonia solanacearum.
Pan Dingwu, Du Huan, Lü Xinyang, Bao Xiaoping . Synthesis and Antibacterial Activities of Novel Quinazoline-2,4-dione Derivatives Containing the 1,2,4-Triazole Schiff-Base Unit[J]. Chinese Journal of Organic Chemistry, 2016 , 36(4) : 818 -825 . DOI: 10.6023/cjoc201510005
[1] Dangi, R. R.; Chundawat, N. S.; Talesara, G. L. World J. Pharm. Res. 2015, 4, 1400.
[2] Wang, D.-W.; Lin, H.-Y.; Cao, R.-J.; Yang, S.-G.; Chen, Q.; Hao, G.-F.; Yang, W.-C.; Yang, G.-F. J. Agric. Food Chem. 2014, 62, 11786.
[3] Wang, D.; Lin H.; Cao, R.; Yang, S.; Chen, T.; He, B.; Chen, Q.; Yang, W.; Yang, G. Acta Chim. Sinica 2015, 73, 29 (in Chinese). (王大伟, 林红艳, 曹润洁, 杨盛刚, 陈涛, 何波, 陈琼, 杨文超, 杨光富, 化学学报, 2015, 73, 29.)
[4] Clark, R. L.; Clements, C. J.; Barrett, M. P.; Mackay, S. P.; Rathnam, R. P.; Owusu-Dapaah, G.; Spencer, J.; Huggan, J. K. Bioorg. Med. Chem. 2012, 20, 6019.
[5] El-Helby, A. G. A. Bull. Pharm. Sci., 2005, 28, 45.
[6] Colotta, V.; Lenzi, O.; Catarzi, D.; Varano, F.; Squarcialupi, L.; Costagli, C.; Galli, A.; Ghelardini, C.; Pugliese, A. M.; Maraula, G.; Coppi, E.; Pellegrini-Giampietro D. E.; Pedata, F.; Sabbadin, D.; Moro, S. Eur. J. Med. Chem. 2012, 54, 470.
[7] Yin, K.; Jiang, L.-H.; Zhou, H.-X.; Huang, Y.; Xiang, J.-N. Chin. J. Org. Chem. 2008, 28, 1016 (in Chinese). (尹凯, 蒋历辉, 周后相, 黄勇, 向建南, 有机化学, 2008, 28, 1016.)
[8] Xiong, Q.; Lin, X.; Liu, J.; Bi, L.; Bao, X. Chin. J. Org. Chem. 2012, 32, 1255 (in Chinese). (熊启中, 林选福, 刘军虎, 毕亮, 鲍小平, 有机化学, 2012, 32, 1255.)
[9] Krishna, K. M.; Inturi, B.; Pujar, G. V.; Purohit, M. N.; Vijaykumar, G. S. Eur. J. Med. Chem. 2014, 84, 516.
[10] Zhu, C.; Wu, F.; Wang, X.; Gao, L.; Weng, Q.; Shi, L.; Zhang, C. Chin. J. Appl. Chem. 2014, 31, 455 (in Chinese). (朱长安, 武飞宇, 王雪, 高丽娜, 翁前锋, 石丽, 张成路, 应用化学, 2014, 31, 455.)
[11] Liu, J.; Liu, Y.; Jian, J.; Bao, X. Chin. J. Org. Chem. 2013, 33, 370 (in Chinese). (刘军虎, 刘勇, 蹇军友, 鲍小平, 有机化学, 2013, 33, 370.)
[12] Li, C.; Liu, J.-C.; Li, Y.-R.; Gou, C.; Zhang, M.-L.; Liu, H.-Y.; Li, X.-Z.; Zheng, C.-J.; Piao, H.-R. Bioorg. Med. Chem. Lett. 2015, 25, 3052.
[13] He, C.; Chen, G. Plant Prot. 2010, 36, 6 (in Chinese). (何晨阳, 陈功友, 植物保护, 2010, 36, 6.)
[14] Taub, B.; Hino, J. B. J. Org. Chem. 1961, 26, 5238.
[15] Sun, X.-H.; Tao, Y.; Liu, Y.-F.; Jia, Y.-Q.; Chen, B. Acta Chim. Sinica 2008, 66, 234 (in Chinese). (孙晓红, 陶燕, 刘源发, 贾婴琦, 陈邦, 化学学报, 2008, 66, 234.)
[16] Wang, X.; Li, P.; Li, Z.; Yin, J.; He, M.; Xue, W.; Chen, Z.; Song, B. J. Agric. Food Chem. 2013, 61, 9575.
/
| 〈 |
|
〉 |