

Chinese Journal of Organic Chemistry >
Progress in Synthesis and Application of Benzo[b][1,4]diazepine Derivatives
Received date: 2015-10-08
Revised date: 2015-11-19
Online published: 2015-12-15
Supported by
Project supported by the National Natural Science Foundation of China (No. 21276064).
Benzo[b][1,4]diazepine derivatives is a class of benzo and seven-number heterocyclic compounds containing double nitrogen atoms. It was concerned for its special structural features, strong physiological and pharmacological activity. Therefore, the research on synthetic method and biological activity of benzo[b][1,4]diazepine has become one of the hot issues in the chemical and pharmaceutical areas. Their application in the antimicrobial, anti-neuroinflammatory, anticancer activity and so on in the recently ten years is reviewed. Besides, the progress in synthesis of the benzo[b][1,4]diazepine compounds at different types of catalysts and its synthetic reaction mechanisms are summarized. Meanwhile, the recent works of authors' research group are discussed. Their synthesis and application prospects are also expected.
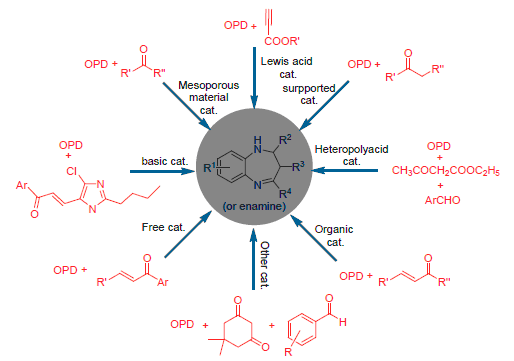
Key words: benzo[b][1,4]diazepine; synthesis; application; catalyst; progress
Yin Liuyan , Wang Lanzhi , Li Xiaoqing , An Yingshuang . Progress in Synthesis and Application of Benzo[b][1,4]diazepine Derivatives[J]. Chinese Journal of Organic Chemistry, 2016 , 36(4) : 711 -723 . DOI: 10.6023/cjoc201510002
[1] (a) Pan, X. Q.; Zou, J. P.; Huang, Z. H.; Zhang, W. Tetrahedron Lett. 2008, 49, 5302.
(b) Li, X. Q.; Wang S. S.; Cheng, S. Y.; Wang, L. Z. J. Hebei Normal Univ. 2013, 37, 317 (in Chinese). (李晓庆, 王莎莎, 程素艳, 王兰芝, 河北师范大学学报, 2013, 37, 317.)
[2] Jiang, Y. J.; Cai, J. J.; Zou, J. P.; Zhang, W. Tetrahedron Lett. 2010, 51, 471.
[3] Wang, L.; Sullivan, G. M.; Hexamer, L. A.; Hasvold, L. A.; Thalji, R.; Przytulinska, M.; Tao, Z. F.; Li, G. Q.; Chen, Z. H.; Xiao, Z.; Gu, W. Z.; Xue, J.; Bui, M. H.; Merta, P.; Kovar, P.; Bouska, J. J.; Zhang, H. Y.; Park, C.; Stewart, K. D.; Sham, H. L.; Sowin, T. J.; Rosenberg, S. H.; Lin, N. H. J. Med. Chem. 2007, 50, 4162.
[4] Corral, C.; Lissavetzky, J.; Valdolmillos, A. M. J. Heterocycl. Chem. 1985, 22, 1349.
[5] Bunin, B. A.; Ellman, J. A. J. Am. Chem. Soc. 1992, 114, 10997.
[6] Reddy, K. V. V.; Rao, P. S.; Ashok, D. Synth. Commun. 2000, 30, 1825.
[7] Bandgar, B. P.; Patil, A. V.; Chavan, O. S. J. Mol. Catal. A: Chem. 2006, 256, 99.
[8] Zhao, H. Y. Ph. D. Dissertation, Doctoral Dissertation of Peking Union Medical College & Chinese Academy of Medical Sciences, Beijing, 2007 (in Chinese). (赵海燕, 博士论文, 中国医学科学院&中国协和医科大学药物研究所, 北京, 2007.)
[9] Wüller, W. E.; Groh, B.; Bub, O.; Hofmann, H. P.; Kreiskott, H. Pharmacopsychiatry 1986, 19, 314.
[10] Kruse, H. Drug Dev. Res. 1982, 2, 145.
[11] Nicholson, A. N.; Stone, B. M.; Clarke, C. H. Br. J. Clin. Pharmacol. 1977, 4, 567.
[12] Al-Hiari, Y. M.; Abu-Dahab, R.; El-Abadelah, M. M. Molecules 2008, 13, 2880.
[13] Konda, S. G.; Shaikh, B. M.; Chavan, S. A.; Dawane, B. S. Chin. Chem. Lett. 2011, 22, 65.
[14] Joshi, Y. C.; Saingar, S.; Kavita; Joshi, P.; Kumar, R. J. Korean Chem. Soc. 2011, 55, 638.
[15] Palanisamy, P.; Jenniefer, S. J.; Muthiah, P. T.; Kumaresan, S. RSC Adv. 2013, 3, 19300.
[16] Thakrar, S.; Bavishi, A.; Radadiya, A.; Parekh, S.; Bhavsar, D.; Vala, H.; Pandya, N.; Shah, A. J. Heterocycl. Chem. 2013, 50, E73.
[17] Wang, L. Z.; Hua, Z. X.; Wang, S. S. Chin. J. Org. Chem. 2013, 33, 2376 (in Chinese). (王兰芝, 花中霞, 王莎莎, 有机化学, 2013, 33, 2376.)
[18] Wang, L. Z.; Li, X. Q.; An, Y. S. Org. Biomol. Chem. 2015, 13, 5497.
[19] Ha, S. K.; Shobha, D.; Moon, E.; Chari, M. A.; Mukkanti, K.; Ki, S. H.; Ahn, K. H.; Kim, S. Y. Bioorg. Med. Chem. Lett. 2010, 20, 3969.
[20] Chen, Y. B.; Le, V.; Xu, X. Y.; Shao, X. S.; Liu, J. W.; Li, Z. Bioorg. Med. Chem. Lett. 2014, 24, 3948.
[21] Ekonomopoulou, M. T.; Tsoleridis, C. A.; Argyraki, M.; Polatoglou, E. Genet. Test. Mol. Biomarkers 2010, 14, 377.
[22] Eleftheriadis, N.; Neochoritis, C. G.; Tsoleridis, C. A.; Julia, S. S.; Zafiroula, I. K. Eur. J. Med. Chem. 2013, 67, 302.
[23] Herbert, J. A. L.; Suschitzky, H. J. Chem. Soc., Perkin. Trans. 1 1974, 2657.
[24] Jung, D. I.; Choi, T. W.; Kim, Y. Y.; Kim, I. S.; Park, Y. M.; Lee, Y. G.; Jung, D. H. Synth. Commun. 1999, 29, 1941.
[25] Balakrishna, M. S.; Kaboudin. B. Tetrahedron Lett. 2001, 42, 1127.
[26] Curini, M.; Epifano, F.; Marcotullio, M. C.; Rosati, O. Tetrahedron Lett. 2001, 42, 3193.
[27] Minothora, P.; Julia, S. S.; Constantinos, A. T. Tetrahedron Lett. 2002, 43, 1755.
[28] Yang, H. Q.; Zhao, S. H.; Liu, Y. Y. Chemistry 2015, 78, 505 (in Chinese). (杨会琴, 赵士豪, 刘月英, 化学通报, 2015, 78, 505.)
[29] Liu, K.; Chen, Y.; Wang, X. J.; Zhang, H. Org.-Fluorine Ind. 2013, 3, 58 (in Chinese). (刘康, 陈越, 王学军, 张恒, 有机氟工业, 2013, 3, 58.)
[30] Nardi, M.; Cozza, A.; Maiuolo, L.; Oliveriob, M.; Procopio, A. Tetrahedron Lett. 2011, 52, 4827.
[31] Wu, H. S.; Yang, J.; Wang, L. Chin. J. Chem. 2011, 29, 1721.
[32] Maiti, G.; Utpal, K.; Karmakar, R.; Bhattacharya, R. N. Tetrahedron Lett. 2012, 53, 1460.
[33] Nagawadea, R. R.; Shinde, D. B. Mendeleev Commun. 2006, 16, 113.
[34] Pawar, S. S.; Shingare, M. S.; Thore, S. N. Chin. Chem. Lett. 2009, 20, 32.
[35] Heravi, M. M.; Zadsirjan, V.; Behbahani, F. K.; Oskooie, H. A. J. Mol. Catal. A: Chem. 2006, 259, 201.
[36] Pandit, S. S.; Vikhe, B. D.; Shelke, G. J. Chem. Sci. 2007, 119, 295.
[37] Rekha, M.; Hamza, A.; Venugopal, B. R.; Nagaraju, N. Chin. J. Catal. 2012, 33, 439.
[38] Chatterjee, N.; Sarkar, S.; Pal, R.; Sen, A. K. Tetrahedron Lett. 2014, 55, 2261.
[39] Guo, P.; Zeng, X. M.; Chen, S.; Luo, M. M. J. Organomet. Chem. 2014, 751, 438.
[40] Bandgar, B. P.; Patl, A. V.; Chavan, O. S. J. Mol. Catal. A: Chem. 2006, 256, 99.
[41] Fazaeli, R.; Aliyan, H. Appl. Catal. A 2007, 331, 78.
[42] Mohammad, A. A.; Iraj, M. B.; Zaghaghi, Z. Catal. Commun. 2008, 9, 2496.
[43] Sharma, R. K.; Gulati, S.; Pandey, A. Inorg. Chim. Acta 2013, 397, 21.
[44] Maleki, A.; Kamalzare, M. Tetrahedron Lett. 2014, 55, 6931.
[45] An, Y. S.; Li, X. Q.; An, X. R.; Wang, L. Z. Monatsh. Chem. 2015, 146, 165.
[46] Wang, D. S.; Yan, L.; Wang, X. L. J. Mol. Catal. 2012, 26, 366 (in Chinese). (王德胜, 闫亮, 王晓来, 分子催化, 2012, 26, 366.)
[47] Heravia, M. M.; Derikvand, F.; Ranjbar, L.; Bamoharram, F. F. J. Mol. Catal. A: Chem. 2007, 261, 156.
[48] Pasquale, G. A.; Ruiz, D. M.; Jios, J. L.; Autinoa, J. C.; Romanelli, G. P. Tetrahedron Lett. 2013, 54, 6574.
[49] Li, X. Q.; Wang, L. Z. Chin. Chem. Lett. 2014, 25, 327.
[50] Das, B.; Ramu, R.; Ravikanth, B.; Reddy, V. S. J. Mol. Catal. A: Chem. 2006, 246, 76.
[51] Kuo, C. W.; More, S. V.; Yao, C. F. Tetrahedron Lett. 2006, 47, 8523.
[52] Sangshetti, J. N.; Kokare, N. D.; Shinde, D. B. Chin. Chem. Lett. 2007, 18, 1305.
[53] Murai, K.; Nakatani, R.; Kita, Y.; Fujioka, H. Tetrahedron 2008, 64, 11034.
[54] Lal, M.; Basha, R. S.; Sarkar, S.; Khan, A. T. Tetrahedron Lett. 2013, 54, 4264.
[55] Yadav, G. D.; Yadav, A. R. Ind. Eng. Chem. Res. 2013, 52, 17812.
[56] Sarkate, A. P.; Sangshetti, J. N.; Dharbale, N. B.; Sarkate, A. P.; Wakte, P. S.; Shinde, D. B. J. Chil. Chem. Soc. 2013, 58, 2200.
[57] Borodina, E. A.; Orlova, N. A.; Kargapolova, I. Y.; Gatilov, Y. V. J. Fluorine Chem. 2014, 162, 66.
[58] Tajbakhsh, M.; Hetavi, M. M.; Mohajerani, B.; Ahmadi, A. N. J. Mol. Catal. A: Chem. 2006, 247, 213.
[59] Vijayasankar, A. V.; Deepa, S.; Venugopal, B. R.; Nagaraju, N. Chin. J. Catal. 2010, 31, 1321.
[60] Shobha, D.; Chari, M. A.; Selvan, S. T.; Oveisib, H.; Manob, A.; Mukkantia, K.; Vinu, A. Microporous Mesoporous Mater. 2010, 129, 112.
[61] Jeganathan, M.; Pitchumani, K. ACS Sustainable Chem. Eng. 2014, 2, 1169.
[62] Yi, W. B.; Cai, C. J. Fluorine Chem. 2009, 130, 1054.
[63] Kurane, R.; Jadhav, J.; Khanapure, S.; Salunkhea, R.; Rashinkar, G. Green Chem. 2013, 15, 1849.
[64] Prakash, G. K. S.; Paknia, F.; Narayan, A.; Mathew, T.; Olah, G. A. J. Fluorine Chem. 2013, 152, 99.
[65] Jadhav, A. H.; Chinnappan, A.; Patil, R. H.; Kostjukb, S. V.; Kim, H. Chem. Eng. J. 2014, 240, 228.
[66] Naeimi, H.; Foroughi, H. Chin. J. Catal. 2015, 36, 734.
[67] Radatz, C. S.; Silva, R. B.; Perin, G.; Lenardão, E. J.; Jacob, R. G.; Alves, D. Tetrahedron Lett. 2011, 52, 4132.
[68] Xu, J. C.; Wei, J. M.; Bian, L. L.; Zhang, J. P.; Chen, J.; Deng, H. M.; Wu, X. Y.; Zhang, H.; Gao, W. Q. Chem. Commun. 2011, 47, 3607.
[69] Christophe, C.; Langlois, B. R.; Billard, T. J. Fluorine Chem. 2013, 155, 118.
[70] Solan, A.; Nisanci, B.; Belcher, M.; Young, J.; Schafer, C.; Wheeler, K. A.; Torok, B.; Dembinski, R. Green Chem. 2014, 16, 1120.
[71] Wang, S. S.; Wang, L. Z. Chem. Res. Appl. 2014, 26, 376 (in Chinese).
/
| 〈 |
|
〉 |