

Chinese Journal of Organic Chemistry >
Synthesis and Photophysical and Electrochemical Properties of 10-Methyl-10H-phenothiazine-5,5-dioxide Derivatives
Received date: 2015-10-09
Revised date: 2015-12-01
Online published: 2015-12-15
Supported by
Project supported by the Science and Technology Development Foundation of Fuzhou University (No. 2010XQ02).
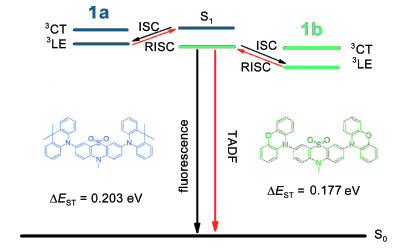
Based on 10-methyl-10H-phenothiazine-5,5-dioxide unit as an acceptor and 9,10-dihydro-9,9-dimethyl-acridine (DMAC) or phenoxazine (PXZ) as donors, four intramolecular charge transfer (ICT) compounds were designed and synthesized by Buchwald-Hartwig cross coupling reactions. Their ground-state geometries were optimized with density functional theory (DFT) at B3LYP level and vertical transition energies were calculated using the optimal Hartree-Fock (HF) exchange method. Resulting compounds were characterized by fourier transform infrared (FT-IR) spectroscopy, nuclear magnetic resonance (NMR) spectroscopy, liquid chromatography-mass (LC-MS) spectrometry and hybrid quadrupole time-of-flight mass spectrometry (QTOF). Their photophysical properties were systematically investigated. The results show that compound 2a has maximum emission wavelength (λmax) of 422 nm in toluene and its oxygen-free one has photoluminescence quantum yield (PLQY) of 0.367. Theλmax and PLQY for 1a and 1b are 455 nm, 0.083 and 511 nm, 0.098, respectively. Transient photoluminescence decay curves for 5 wt% 1a and 1b doped in PMMA films indicate that their delayed fluorescence lifetimes are 5.78 and 20.00 μs, respectively. According to the time-resolved fluorescence and phosphorescence spectra of 1a and 1b doped in PMMA films at 77 K, their energy gaps (ΔEST) between the lowest singlet state (S1) and the lowest triplet excited state (T1) are determined to be 0.203 and 0.177 eV, respectively, which are consistent with the theoretical values 0.232 and 0.295 eV calculated with time-dependent density functional theory (TD-DFT). As a result, 1a and 1b are expected to be potential thermally activated delayed fluorescence (TADF) materials. The electrochemical properties of the four compounds in dichloromethane solution were determined through cyclic voltammetry using TBAPF6 as supporting electrolyte and ferrocene as internal standard. The HOMO energy levels of 1a, 1b, 2a and 2b are estimated to be -5.369, -5.111, -5.412 and -5.075 eV, respectively. The thermal properties of the four compounds were investigated by thermal gravimetric analysis under nitrogen atmosphere at a heating rate of 10 ℃·min-1 and all compounds show good thermal stability with decomposition temperatures above 420 ℃.
Zheng Yueyou , Xie Qionglin , Wang Bingxi . Synthesis and Photophysical and Electrochemical Properties of 10-Methyl-10H-phenothiazine-5,5-dioxide Derivatives[J]. Chinese Journal of Organic Chemistry, 2016 , 36(4) : 803 -811 . DOI: 10.6023/cjoc201510008
[1] Huang, C. H.; Li, F. Y.; Huang, W. Introduction to Organic Light-emitting Materials and Devices, Fudan University Press, Shanghai, 2005, pp. 3~4 (in Chinese). (黄春辉, 李富友, 黄维, 有机电致发光材料与器件导论, 复旦大学出版社, 上海, 2005, pp. 3~4.)
[2] Lowry, M. S.; Bernhard, S. Chem. Eur. J. 2006, 12, 7974.
[3] Balzani, V.; Juris, A. Coord. Chem. Rev. 2001, 211, 100.
[4] Zhang, Q. S.; Li, J.; Shizu, K.; Huang, S. P.; Hirata, S.; Miyazaki, H.; Adachi, C. J. Am. Chem. Soc. 2012, 134, 14707.
[5] Wu, S. H.; Aonuma, M.; Zhang, Q. S.; Huang, S. P.; Nakagawa, T.; Kuwabara, K.; Adachi, C. J. Mater. Chem. C 2014, 2, 422.
[6] Zhang, Q. S.; Li, B.; Huang S. P.; Nomura, H.; Tanaka, H.; Adachi, C. Nat. Photonics 2014, 8, 326.
[7] Huang, B.; Qi, Q.; Jiang, W.; Tang, J.; Liu, Y. Y.; Fan, W. J.; Yin, Z. H.; Shi, F.; Ban, X. X.; Xu, H. G.; Sun, Y. M. Dyes Pigm. 2014, 111, 135.
[8] Wang, H.; Xie, L. S.; Peng. Q.; Meng, L. Q.; Wang, Y.; Yi, Y. P.; Wang, P. F. Adv. Mater. 2014, 26, 5201.
[9] Zhang, Q. S.; Kuwabara, H.; J. Potscavage, Jr, W.; Huang, S. P.; Hatae, Y.; Shibata, T.; Adachi, C. J. Am. Chem. Soc. 2014, 136, 18076.
[10] Wang, F. F.; Tao, Y. T.; Huang, W. Acta Chim. Sinica 2015, 73, 11 (in Chinese). (王芳芳, 陶友田, 黄维, 化学学报, 2015, 73, 11.)
[11] Huang, S. P.; Zhang, Q. S.; Shiota, Y.; Nakagawa, T.; Kuwabra, K.; Yoshiazawa, K.; Adachi, C. J. Chem. Theory Comput. 2013, 9, 3874.
[12] Chen, J. X.; Huang, X. W. Organic Electroluminescent Materials & Devices, Tsinghua University Press, Beijing, 2005, p. 14 (in Chinese). (陈金鑫, 黄孝文, OLED有机电致发光材料与器件, 清华大学出版社, 北京, 2005, p. 14.)
[13] Huang, B.; Dai, Y.; Ban, X. X.; Jiang, W.; Zhang, Z. H.; Sun, K. Y.; Lin, B. P.; Sun, Y. M. Acta Phys.-Chim. Sin. 2015, 31, 1627 (in Chinese). (黄斌, 代钰, 班鑫鑫, 蒋伟, 张兆杭, 孙开涌, 林保平, 孙岳明, 物理化学学报, 2015, 31(8), 1627.)
[14] Lee, J. Y.; Shizu, K.; Tanaka, H.; Nomura, H.; Yasuda, T.; Adachi, C. J. Mater. Chem. C 2013, 1, 4599.
[15] Shaheen, S.; Jabbour, G.; Kippelen, B.; Peyghambarian, N.; Anderson, J.; Marder, S.; Armstrong, N.; Bellmann, E.; Grubbs, R. Appl. Phys. Lett. 1999, 74, 3212.
[16] Xiang, N. J.; Li, D. H.; Liang, W. L.; Su, S. J.; Shi, J. X.; Gong, M. L. Acta Chim. Sinica 2006, 64, 1158 (in Chinese). (向能军, 李狄豪, 梁万里, 苏树江, 石建新, 龚孟濂, 化学学报, 2006, 64, 1158.)
[17] Aan, H. C. WO 2012039561, 2012 [Chem. Abstr. 2012, 156, 493689].
[18] Deronzier, A. WO 2012066048, 2012 [Chem. Abstr. 2012, 156, 671582].
[19] Kim, G.; Yeom, H.; Cho, S.; Seo, J.; Kim, J.; Yang, C. Macromolecules 2012, 45, 1850.
/
| 〈 |
|
〉 |