

Chinese Journal of Organic Chemistry >
Synthesis and Anti-tumor Activity of Derivatives of Ring A of Ursolic Acid
Received date: 2015-10-28
Revised date: 2015-12-16
Online published: 2016-01-04
Supported by
Project supported by the National Natural Science Foundation of China (No. 21372156), the Education Department of Higher School Outstanding Talent Support Program in Liaoning Province (No. LR2013017) and the Science and Technology Project in Shenyang (No. F 16-230-6-00) .
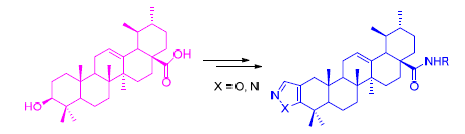
Ten derivatives were successfully synthesized by the introduction of nitrogen-and oxygen-containing heterocyclic compounds at ring A and the modification of the amidation at C(28) position in the natural product ursolic acid, which structures were characterized by 1H NMR, 13C NMR, MS and elemental analysis. Their anti-tumor activities against KB and SGC7901 cells in vitro were evaluated by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. These results indicated that all of derivatives can inhibit cell proliferation of KB and SGC7901 cells better than ursolic acid (UA), and UA4-1 (IC50=0. 289 μmol/L) and UA4-2 (IC50=0. 04 μmol/L) were also found to have especially more potent inhibitory activity on SGC7901 tumor cells than that of the positive control drug gefitinib (IC50=1. 03 μmol/L).
Key words: pentacyclic trierpenoid; ursolic acid; anti-tumor activity
Meng Yanqiu , Cao Jia , Tang Yi , Lu Xueyu , Liu Liwei . Synthesis and Anti-tumor Activity of Derivatives of Ring A of Ursolic Acid[J]. Chinese Journal of Organic Chemistry, 2016 , 36(5) : 1080 -1087 . DOI: 10.6023/cjoc201510034
[1] Wang, P.; Zhang, Z. Y.; Wu, Z. J. Chin. Med. Mater. 2000, 23, 717.
[2] Kazakova, O. B.; Giniyatullina, G. V.; Yamansarov, E. Y.; Tolstikov, G. A. Bioorg. Med. Chem. Lett. 2010, 20, 4088.
[3] Cunha, W. R.; de Matos, G. X.; Souza, M. G.; Tozatti, M. G.; Andrade, E.; Silva, M. L.; Martins, C. H.; da Silva, R.; Da Silva Filho, A. A. Pharm. Biol.2010, 48, 166.
[4] Jin, Y. R.; Jin, J. L.; Li, C. H.; Piao, X. X.; Jin, N. G. Pharm. Biol. 2012, 50, 523.
[5] Liu, W.; Tan, X.; Shu, L.; Sun, H.; Song, J.; Jin, P.; Yu, S.; Sun, M.; Jia, X. Molecules 2012, 17, 9104.
[6] Wang, J.; Liu, L.; Qiu, H.; Zhang, X.; Guo, W.; Chen, W.; Tian, Y.; Fu, L.; Shi, D.; Cheng, J.; Huang, W.; Deng, W. PLoS One 2013, 8, 63872.
[7] Leal, A. S.; Wang, R.; Salvador, J. A.; Jing, Y. Bioorg. Med. Chem. 2012, 20, 5774.
[8] Nobuchika, S.; Kouhei, T.; Nicole, H.; Kenji, D.; Reiko, T.; Shiro, M. J. Biol. Chem. 2009, 284, 5000.
[9] Meng, Y. Q.; Zhan, L. F. J. Asian Nat. Prod. Res. 2015, 11, 1.
[10] Fu, H. J.; Zhou, Y. R.; Bao, B. H.; Jia, M. X.; Zhao, Y.; Zhang, L.; Li, J. X.; He, H. L.; Zhou, X. M. J. Med. Chem. 2014, 57, 4692.
[11] Liu, D.; Meng, Y. Q.; Zhao, J. Chem. Res. Chin. Univ. 2008, 24, 42.
[12] Shen, L. H.; Lai, Y. S.; Zhang, Y. H. J. Chin. Med. Univ. 2008, 39, 103 (in Chinese).
(申利红, 赖宜生, 张奕华, 中国药科大学学报, 2008, 39, 103.)
[13] Bi, Y.; Xu, J. Y.; Wu, X. M. Chin. J. New Drugs 2005, 14, 23 (in Chinese).
(毕毅, 徐进宜, 吴晓明, 中国新药杂志, 2005, 14, 23.)
[14] Li, D.; Zhou, J. P.; Wu, X. M. Pharm. Dev. 2004, 28, 120 (in Chinese).
(李丹, 周金培, 吴晓明, 药学进展, 2004, 28, 120.)
[15] Meng, Y. Q. Bioorg. Med. Chem. 2009, 17, 848.
[16] Chen, J.; Fu, H.; Wang, Z.; Yin, F.; Li, J.; Hua, Y. Cell. Phys. Biochem. 2014, 34, 724.
[17] Fu, H. J.; Yang, Z.; Zhou, Y. R.; Bao, B. H.; Yun, D.; Li, J. X. Eur. J. Pharm. Sci. 2015, 76, 33.
[18] Chen, J.; Liu, J.; Zhang, L. Y.; Wu, G. Z.; Hua, W. Y.; Wu, X. M.; Sun, H. B. Bioorg. Med. Chem. Lett. 2006, 16, 2915.
[19] Qiu, W. W.; Shen, Q.; Yang, F.; Wang, B.; Zou, H.; Li, J. Y.; Li, J.; Tang, J. Bioorg. Med. Chem. Lett. 2009, 19, 6618.
/
| 〈 |
|
〉 |