

Chinese Journal of Organic Chemistry >
Synthesis and Biological Activities of Novel Triazolesulfonamide Derivatives Containing Quinolin-2-one Moiety
Received date: 2015-10-12
Revised date: 2015-11-30
Online published: 2016-01-07
Supported by
Project supported by the National Natural Science Foundation of China (No. 21272091), the Hubei Provincial Department of Education (No. Q20102606), the Fundamental Research Funds for the Central Universities (No. CCNU15A02014), the Xiangyang Science and Technology Bureau (No. 2010GG1B33), and the Natural Science Foundation of Hubei Province (No. 2014CFC1153).
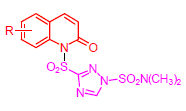
Complex III is an essential component for cellular respiration chain and one of the most important targets of fungicides. According to bioisosterism and combination of bioactive sub-structive, a series of novel triazolesulfonamide derivatives containing quinolin-2-one moiety were designed and synthesized. The structures of the synthesized compounds were confirmed by MS, 1H NMR, 13C NMR and elemental analysis. The antibacterial activities of these compounds against porcine complex III were evaluated, and the bioassay results indicated that the target compound 6f [IC50=(45.75±1.02) μmol/L] showed much higher inhibitory potency (more than one-fold higher) against complex III than that of the control amisulbrom [IC50=(92.96±1.30) μmol/L], thus compound 6f may recognize as a lead for further structure modification.
Cheng Hua , Wang Wanqiang , Huang Lin , Cui Ping , Wu Qiongyou . Synthesis and Biological Activities of Novel Triazolesulfonamide Derivatives Containing Quinolin-2-one Moiety[J]. Chinese Journal of Organic Chemistry, 2016 , 36(5) : 1065 -1072 . DOI: 10.6023/cjoc201510013
[1] (1) Sun, F.; Zhou, Q. J.; Sun, J.; Zhai, Y. J.; Rao, Z. H. Bull. Life Sci.2008, 20(4), 566 (in Chinese).
(孙飞, 周强军, 孙吉, 翟宇佳, 饶子和, 生命科学, 2008, 20(4), 566.)
[2] Zhang, Z. L.; Lishar, H.; Shulmeister, V. M.; Chi, Y. I.; Kyeong, K. K.; Hung, L. W.; Crofts, A. R.; Berry, E. A.; Kim, S. H. Nature 1998, 392, 677.
[3] Iwata, S.; Lee, J. W.; Okada, K.; Lee, J. K.; Iwata, M.; Rasmussen, B.; Link, T. A.; Ramaswamy, S. Science 1998, 281, 64.
[4] Hunte, C.; Koepke, J.; Lange, C.; Romanith, T.; Michel, H. Structure 2000, 8(6), 669.
[5] Gao, X. G; Wen, X. L.; Esser, L.; Quinn, B.; Yu, L. D.; Yu, C. A.; Xia, D. Biochemistry 2003, 42(30), 9067.
[6] Esser, L.; Quinn, B.; Li, Y. F.; Zhang, M. M.; Elberry, L.; Yu, C. A.; Xia, D. J. Mol. Biol. 2004, 341(1), 281.
[7] Huang, L.; Cobessi, D.; Tung, E. Y.; Berry, E. A. J. Mol. Biol. 2005, 351(3), 573.
[8] Yang, W. C.; Li, H.; Wang, F.; Zhu, X. L.; Yang, G. F. ChemBioChem 2012, 13(11), 1542.
[9] Maklashina, E.; Cecchini, G. Biochim. Biophys. Acta 2010, 1797(12), 1877.
[10] Li, H.; Zhu, X. L.; Yang, W. C.; Yang, G. F. Chem. Biol. Drug. Des. 2014, 83(1), 71.
[11] Cheng, H.; Shen, Y. Q.; Pan, X. Y.; Hou, Y. P.; Wu, Q. Y.; Yang, G. F. New J. Chem. 2015, 39(9), 7281.
[12] Tang, J. L.; Xu, J. G.; Song, J.; Guang, L.; Chen, L. J. Guangdong Pharm. Univ. 2013, 29(6), 596 (in Chinese).
(汤洁丽, 许俊耿, 宋健, 关丽, 陈琳, 广东药学院学报, 2013, 29(6), 596.)
[13] Harper, S.; Avolio, S. J. Med. Chem. 2005, 48(2), 547.
[14] Oshiro, Y.; Sam, S. J. Med. Chem. 1998, 41(5), 658.
[15] Salmon, R.; Mitchell, G.; Morris, J. A. WO 2010116122, 2010[Chem. Abstr. 2010, 153, 470191].
[16] Yang, J. Q.; Hu, Y. W.; Gu, Q.; Li, M. G.; Li, M. Q.; Song, B. A. Chin. J. Org. Chem. 2014, 34, 829.
(in Chinese) (杨家强, 胡月维, 谷晴, 李明刚, 李明强, 宋宝安, 有机化学, 2014, 34, 829.)
[17] Mahieu, J. P.; Gosselet, M.; Sebille, B.; Beuzard, Y. Synth. Commun. 1986, 16(13), 1709.
[18] Manimaran, T.; Thiruvengadam, T. K.; Ramakrishnan, V. T. Synth. Commun. 1975, 11, 739.
[19] Hegde, S. G.; Mahoney, M. D. WO 2000042028, 2000[Chem. Abstr.2000, 133, 105038].
[20] Fukuda, K.; Kondo, Y.; Makabe, T.; Tanaka, N.; Utsunomiya, T.; Sakurai, Y. WO 2006057354, 2006[Chem. Abstr.2006, 145, 27993].
/
| 〈 |
|
〉 |