

Chinese Journal of Organic Chemistry >
Preparation of 1,3,5-Trisubstituted Pyrazoles with Cascade Reaction Catalyzed by Cu between Hydrazonoyl Halide and Terminal Alkynes
Received date: 2015-11-18
Revised date: 2015-12-22
Online published: 2016-01-07
Supported by
Project supported by the National Natural Science Foundation of China (No. 81302650).
Cascade reaction, including nucleophilic substitution and addition cyclization, catalyzed by Cu+ salt between substituted aryl hydrazonoyl halides and terminal alkynes could produce 1,3,5-trisubstituted pyrazoles. The method employed easily available aryl hydrazonoyl halide and terminal alkynes as starting materials under 45 ℃ in green acetonitrile/water solvent, regioselectively generating 1,3,5-trisubstituted pyrazoles in high yield. Above all, functional groups containing active hydrogen would not disturb this reaction, example for carboxyl and hydroxyl. In this work 17 pyrazole derivatives with different substituent group were achieved by this method. So it could be a general method to synthesize 1,3,5-trisubstituted pyrazoles.
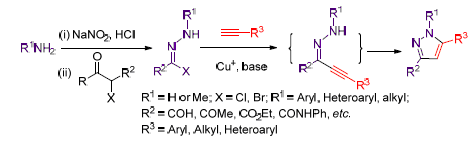
Li Fuwei , Wang Xiaolong , Yu Haitao . Preparation of 1,3,5-Trisubstituted Pyrazoles with Cascade Reaction Catalyzed by Cu between Hydrazonoyl Halide and Terminal Alkynes[J]. Chinese Journal of Organic Chemistry, 2016 , 36(5) : 1127 -1132 . DOI: 10.6023/cjoc201511033
[1] (a) Elguero, J.; Pilar, J.; Nadine, S.; Artur, M. S. Targets Heterocycl. Syst. 2002, 6, 52.
(b) Bondock, S.; Fadaly, W.; Metwally, M. A. Eur. J. Med. Chem. 2010, 45, 3692.
(c) Barsoum, F. F.; Girgis, A. S. Eur. J. Med. Chem. 2009, 44, 2172.
(d) Manfredini, S.; Bazzanini, R.; Baraldi, P. G.; Guarneri, M. J. Med. Chem. 1992, 35, 917.
[2] (a) Rudorf, W. D.; Augustin, M. J. Prakt. Chem. 1978, 320, 585.
(b) Battistone, M. J.; Sawitzke, A. D. Clin. Med. Insights: Ther. 2010, 2, 245.
[3] Akula, R.; Suresh, B. M.; Ramamohan, M.; Raghavendra, K. R.; Thalishetti, K,; Gangadhara, R. C.; Vaikunta, L. R. Tetrahedron Lett. 2014, 55, 2986.
[4] (a) Rosa, F. A.; Machado, P.; Vargas, P. S.; Bonacorso, H. G.; Zanatta, N.; Martins, M. A. P. Synlett 2008, 1673.
(b) Giacomelli, G.; Porcheddu, A.; Salaris, M.; Taddei, M. Eur. J. Org. Chem. 2003, 537.
(c) Donnohue, B. A.; Michelotti, E. L.; Reader, J. C.; Reader, V.; Stirling, M.; Tice, C. N. J. Comb. Chem. 2002, 4, 23.
[5] (a) Clovis, J. S.; Fliege, W.; Huisgen, R. Chem. Ber. 1983, 116, 3062.
(b) Huisgen, R.; Seidel, M.; Wallbillich, G. Tetrahedron1962, 17, 3.
(c) Fliege, W.; Grashey, R.; Huisgen, R. Chem. Ber. 1984, 117, 1194.
[6] (a) Liang, D. D.; Zhu, Q. Asian J. Org. Chem. 2015, 4, 42.
(b) Li, X.; He, L.; Jiang, H. J. Org. Chem. 2013, 78, 3636.
(c) Hu, J.; Chen, S.; Sun, Y.; Yang, J.; Rao, Y. Org. Lett. 2012, 14, 5030.
(d) Zhang, T.; Bao, W. J. Org. Chem. 2013, 78, 1317.
[7] Browne, D. L.; Taylor, J. B.; Plant, A.; Harrity, J. P. A. J. Org. Chem. 2010, 75, 984.
[8] Ahmed, M. S. M.; Kobayashi, K.; Mori, A. Org. Lett. 2005, 7, 4487.
[9] (a) Longhi, K.; Moreira, D. N.; Marzari, M. R. B.; Floss, V. M.; Bonacorso, H. G.; Zanatta, N.; Martins, M. A. P. Tetrahedron Lett. 2010, 51, 3193.
(b) Bhat, B. A.; Puri, S. C.; Qurishi, M. A.; Dhar, K. L.; Qazi, G. N. Synth. Commun. 2005, 35, 1135.
[10] Zara, M.; Kivrak, A. J. Org. Chem. 2011, 76, 9379.
[11] Sun, A. X.; Ye, J. H.; Yu, H. T.; Zhang, W. C.; Wang, X. L. Tetrahedron Lett. 2014, 55, 889.
[12] (a) Rostovtsev, V.; Green, L.; Fokin, V.; Sharpless, K. Angew. Chem., Int. Ed. 2002, 41, 2596.
(b) Tornoe, C.; Christensen, C.; Meldel, M. J. Org. Chem. 2002, 67, 3057.
(c) Brik, A.; Muldoon, J.; Lin, Y.; Elder, J. ChemBioChem 2003, 4, 1246.
(d) Chan, T. R.; Hilgraf, R.; Shapless, K. B.; Fokin, V. V. Org. Lett. 2004, 6, 2853.
(e) Wang, Q.; Chan, T. R.; Hilgraf, R.; Fokin, V. V.; Shapless, K. B.; Finn, M. G. J. Am. Chem. Soc. 2003, 125, 3192.
[13] (a) Faucher, N. E.; Martres, P. WO 2005049578, 2005 [Chem. Abstr. 2005, 143, 26597].
(b) Silvestri, R.; Cascio, M. G.; Giuseppe, L. R.; Piscitelli, F.; Lavecchia, A.; Brizzi, A.; Pasquini, S.; Botta, M.; Novellino, E.; Vincenzo, D. M.; Corelli, F. J. Org. Chem. 2008, 51, 1560.
/
| 〈 |
|
〉 |