

Chinese Journal of Organic Chemistry >
Facile Synthesis of Ferrocenylchalcone Promoted by Ionic Liquid or Phase-Transfer Catalyst
Received date: 2015-11-25
Revised date: 2015-12-14
Online published: 2016-01-15
Supported by
Project supported by the National Natural Science Foundation of China (No. 21562032), the Research Program of Science and Technology at Universities of Inner Mongolia (No. NJZZ001) and the Natural Science Foundation of Inner Mongolia (Nos. 2013MS0207, 2014JQ02).
The Claisen-Schmidt condensation of acetylferrocene with arylaldehydes in the presence of ionic liquids (ILs) or phase-transfer catalysts (PTCs) provided the corresponding ferrocenylchalcones in high yields, and PTCs can promote the condensation reaction better than those of ILs. Optimum conditions were as follows: molar ratio of acetylferrocene, arylaldehyde, n-Bu4NPF6 and NaOH is 1:1:2.5:0.75, and the appropriate reaction temperature is 35 ℃ in anhydrous EtOH.
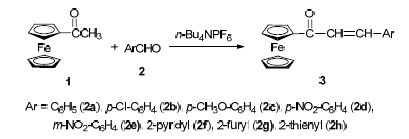
Zhao Haiying , Yin Fengnan , Yu Lingyan , Li Baoguo , Bian Zhanxi . Facile Synthesis of Ferrocenylchalcone Promoted by Ionic Liquid or Phase-Transfer Catalyst[J]. Chinese Journal of Organic Chemistry, 2016 , 36(5) : 1118 -1121 . DOI: 10.6023/cjoc201511045
[1] Patil, P. S.; Dharmaprakash, S. M.; Ramakrishna, K.; Fun, H. K. J. Cryst. Growth 2007, 303, 520.
[2] Zhao, H.; Zhu, X.; Wang, D.; Chen, S.; Bian, Z. Aust. J. Chem. 2015, 68, 1035.
[3] Avila, H.; Smania, E.; Monache, F.; Junior, A. Bioorg. Med. Chem. 2008, 16, 9790.
[4] Niu, C.; Li, G.; Tuerxuntayi, A.; Aisa, H. A. Chin. J. Chem. 2015, 33, 486.
[5] Prasath, R.; Bhavana, P.; Ng, S. W.; Tiekink, E. R. T. J. Organomet. Chem. 2013, 726, 62.
[6] Attar, S.; O'Brien, Z.; Alhaddad, H.; Golden, M. L.; Calderón-Urrea, A. Bioorg. Med. Chem. 2011, 19, 2055.
[7] Wu, X.; Tiekink, E. R. T.; Kostetski, I.; Kocherginsky, N.; Tan, A. L. C.; Khoo, S. B.; Wilairat, P.; Go, M. L. Eur. J. Pharm. Sci. 2006, 27, 175.
[8] Wang, D.; Zhu, X. Y.; Zhao, H. Y.; Bian, Z. X. Chin. J. Org. Chem. 2015, 35, 1131 (in Chinese).
(王栋, 朱学友, 赵海英, 边占喜, 有机化学, 2015, 35, 1131.)
[9] Yang, J. M.; Ji, S. J.; Gu, D. G.; Shen, Z. L.; Wang, S. Y. J. Organomet. Chem. 2005, 690, 2989.
[10] Parveen, H.; Hayat, F.; Salahuddin, A.; Azam, A. Eur. J. Med. Chem. 2010, 45, 3497.
[11] Gong, Z. L.; Xie, Y. S; Zhao, B. X.; Lv, H. S.; Liu, W. Y.; Zheng, L. W.; Lian, S. J. Fluoresc. 2011, 21, 355.
[12] Bukhari, S. N. A.; Jasamai, M.; Jantan, I.; Ahmad, W. Mini-Rev. Org. Chem. 2013, 10, 73.
[13] Fang, D.; Cheng, J.; Fei, Z.; Gong, K.; Liu, Z. Catal. Commun. 2008, 9, 1924.
[14] Shibata, K.; Katsuyama, I.; Matsui, M.; Muramatsu, H. Bull. Chem. Soc. Jpn. 1990, 63, 3710.
[15] Erasmus, E. Inorg. Chim. Acta 2011, 378, 95.
[16] Abashev, G. G.; Antuf'eva, A. D.; Bushueva, A. Y.; Kudryavtsev, P. G.; Osorgina, I. V.; Syutkin, R. V.; Shklyaeva, E. V. Russ. J. Appl. Chem. 2010, 83, 1435.
[17] Muller, T. J.; Conradie, J.; Erasmus, E. Polyhedron 2012, 33, 257.
[18] Srivastava, Y. K. Rasayan J. Chem. 2008, 1, 884.
[19] Liu, Y. T.; Lian, G. D.; Yin, D. W.; Su, B. J. Res. Chem. Intermed. 2012, 38, 1043.
[20] Liu, Y. T.; Feng, L.; Yin, D. W. Res. Chem. Intermed. 2013, 39, 2971.
[21] Ji, S. J.; Wang, S. Y.; Shen, Z. L.; Zhou, M. F. Chin. Chem. Lett. 2003, 14, 1246.
[22] Liu, W.; Xu, Q.; Chen, B.; Ma, Y. Synth. Commun. 2002, 32, 171.
[23] Salisova, M.; Puciova, M.; Postnov, U. N.; Toma, S. Chem. Papers 1990, 44, 201.
[24] Villemin, D.; Martin, B.; Puciova, M.; Toma, S. J. Organomet. Chem. 1994, 484, 27.
/
| 〈 |
|
〉 |