

Chinese Journal of Organic Chemistry >
Development of the Asymmetric Hydrogenation of Enol Esters
Received date: 2015-12-07
Revised date: 2016-01-06
Online published: 2016-01-15
Supported by
Project supported by the National Natural Science Foundation of China (No. 21572131) and the Science and Technology Commission of Shanghai Municipality (No. 14XD1402300).
Chiral alcohols are an important class of compounds and possess a broad array of applications, thus their asymmetric preparation is an important area of research in the field of organic synthesis. Amongst methodologies for the preparation of such compounds, catalytic asymmetric hydrogenation has gained widespread interest due to its efficiency, environmentally friendliness and economic advantages, and is gradually becoming a technology with great potential for the industrial preparation of chiral alcohols. This review provides the first overview for the catalytic asymmetric hydrogenation of enol esters for the synthesis of chiral alcohols. A comprehensive and up-to-date introduction are given for a number of different substrates. A thorough analysis is provided concerning the advantages and disadvantages of different chiral ligands and their transition-metal complexes. Finally, a brief discussion relating to developments and potential areas of further research concerning new substrates, new ligands and new catalytic metals, is presented.
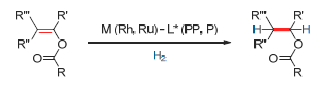
Key words: enol esters; chiral alcohols; asymmetric hydrogenation; Ru-catalyzed; Rh-catalyzed
Wang Zhihui , Zhang Zhenfeng , Liu Yangang , Zhang Wanbin . Development of the Asymmetric Hydrogenation of Enol Esters[J]. Chinese Journal of Organic Chemistry, 2016 , 36(3) : 447 -459 . DOI: 10.6023/cjoc201512009
[1] (a) de Vries, J. G.; Elsevier, C. J. Handbook of Homogeneous Hydrogenation, Wiley-VCH, Weinheim, Germany, 2007.
(b) Ma, Y.; Zhang, Y. J.; Zhang, W. Chin. J. Org. Chem. 2007, 27, 289 (in Chinese). (马元辉, 张勇健, 张万斌, 有机化学, 2007, 27, 289.)
(c) Blaser, H.-U.; Federsel, H.-J. Asymmetric Catalysis on Industrial Scale, 2nd ed., Wiley-VCH, Weinheim, Germany, 2010.
(d) Xie, J.-H.; Zhou, Q.-L. Acta Chim. Sinica 2012, 70, 1427 (in Chinese). (谢建华, 周其林, 化学学报, 2012, 70, 1427.)
(e) Etayo, P.; Vidal-Ferran, A. Chem. Soc. Rev. 2013, 42, 728.
(f) Wang, Y.; Zhang, Z.; Zhang, W. Chin. J. Org. Chem. 2015, 35, 528 (in Chinese). (王英杰, 张振锋, 张万斌, 有机化学, 2015, 35, 528.)
[2] (a) Noyori, R.; Ohkuma, T. Angew. Chem., Int. Ed. 2001, 40, 40.
(b) Klingler, F. D. Acc. Chem. Res. 2007, 40, 1367.
[3] Fryzuk, M. D.; Bosnich, B. J. Am. Chem. Soc. 1978, 100, 5491.
[4] Koenig, K. E.; Bachman, G. L.; Vineyard, B. D. J. Org. Chem. 1980, 45, 2362.
[5] Brown, J. M.; Murrer, B. A. J. Chem. Soc., Perkin Trans. 2 1982, 489.
[6] Burk, M. J. J. Am. Chem. Soc. 1991, 113, 8518.
[7] Lotz, M.; Ireland, T.; Perea, J. J. A.; Knochel, P. Tetrahedron: Asymmetry 1999, 10, 1839.
[8] (a) Ireland, T.; Tappe, K.; Grossheimann, G.; Knochel, P. Chem. Eur. J. 2002, 8, 843.
(b) Lotz, M.; Polborn, K.; Knochel, P. Angew. Chem., Int. Ed. 2002, 41, 4708.
[9] Gavrilov, K. N.; Benetsky, E. B.; Boyko, V. E.; Rastorguev, E. A.; Davankov, V. A.; Schäffner, B.; Börner, A. Chirality 2010, 22, 844.
[10] Schmidt, U.; Langner, J.; Kirschbaum, B.; Braun, C. Synthesis 1994, 1138.
[11] Lüttenberg, S.; Ta, T. D.; von der Heyden, J.; Scherkenberk, J. Eur. J. Org. Chem. 2013, 1824.
[12] (a) Stephan, M.; Šterk, D.; Mohar, B. Adv. Synth. Catal. 2009, 351, 2779.
(b) Zupan?i?, B.; Mohar, B.; Stephan, M. Org. Lett. 2010, 12, 1296.
(c) Zupan?i?, B.; Mohar, B.; Stephan, M. Org. Lett. 2010, 12, 3022.
[13] Burk, M. J.; Kalberg, C. S.; Pizzano, A. J. Am. Chem. Soc. 1998, 120, 4345.
[14] Han, X.; Jiang, X.-J.; Civiello, R. L.; Degnan, A. P.; Chaturvedula, P. V.; Macor, J. E.; Dubowchik, G. M. J. Org. Chem. 2009, 74, 3993.
[15] Liu, Y.; Sandoval, C. A.; Yamaguchi, Y.; Zhang, X.; Wang, Z.; Kato, K.; Ding, K. J. Am. Chem. Soc. 2006, 128, 14212.
[16] (a) Dong, C.; Wang, Y.; Zhu, Y. Z. Bioorg. Med. Chem. 2009, 17, 3499.
(b) Pan, L.-L.; Wang, J.; Jia, Y.-L.; Zheng, H.-M.; Wang, Y.; Zhu, Y.-Z. Int. J. Mol. Sci. 2015, 16, 628.
[17] Wang, Q.; Huang, W.; Yuan, H.; Cai, Q.; Chen, L.; Lv, H.; Zhang, X. J. Am. Chem. Soc. 2014, 136, 16120.
[18] Boaz, N. W. Tetrahedron Lett. 1998, 39, 5505.
[19] Li, W.; Zhang, Z.; Xiao, D.; Zhang, X. J. Org. Chem. 2000, 65, 3489.
[20] Tang, W.; Liu, D.; Zhang, X. Org. Lett. 2003, 5, 205.
[21] Liu, D.; Zhang, X. Eur. J. Org. Chem. 2005, 646.
[22] Zhang, X.; Huang, K.; Hou, G.; Cao, B.; Zhang, X. Angew. Chem., Int. Ed. 2010, 49, 6421.
[23] Pullmann, T.; Engendahl, B.; Zhang, Z.; Hölscher, M.; Zanotti-Gerosa, A.; Dyke, A.; Franciò, G.; Leitner, W. Chem. Eur. J. 2010, 16, 7517.
[24] Knorad, T. M.; Schmitz, P.; Leitner, W.; Franciò, G. Chem. Eur. J. 2013, 19, 13299.
[25] Robert, T.; Abiri, Z.; Sandee, A. J.; Schmalz, H.-G.; Reek, J. N. H. Tetrahedron: Asymmetry 2010, 21, 2671.
[26] (a) Etayo, P.; Núñez-Rico, J. L.; Fernández-Pérez, H.; Vidal-Ferran, A. Chem. Eur. J. 2011, 17, 13978.
(b) Etayo, P.; Núñez-Rico, J. L.; Vidal-Ferran, A. Organometallics 2011, 30, 6718.
(c) Núñez-Rico, J. L.; Etayo, P.; Fernández-Pérez, H.; Vidal-Ferran, A. Adv. Synth. Catal. 2012, 354, 3025.
[27] (a) Fernández-Pérez, H.; Benet-Buchholz, J.; Vidal-Ferran, A. Org. Lett. 2013, 15, 3634.
(b) Fernández-Pérez, H.; Benet-Buchholz, J.; Vidal-Ferran, A. Chem. Eur. J. 2014, 20, 15375.
(c). Lao, J. R. Benet-Buchholz, J.; Vidal-Ferran, A. Organometallics 2014, 33, 2960.
[28] (a) Kleman, P.; González-Liste, P. J.; García-Garrido, S. E.; Cadierno, V.; Pizzano, A. Chem. Eur. J. 2013, 19, 16209.
(b) Kleman, P.; González-Liste, P. J.; García-Garrido, S. E.; Cadierno, V.; Pizzano, A. ACS. Catal. 2014, 4, 4398.
[29] Reetz, M. T.; Goossen, L. J.; Meiswinkel, A.; Paetzold, J.; Jensen, J. F. Org. Lett. 2003, 5, 3099.
[30] Mamone, P.; Grünberg, M. F.; Fromm, A.; Khan, B. A.; Gooβen, L. J. Org. Lett. 2012, 14, 3716.
[31] Alegre, S.; Alberico, E.; Pàmies, O.; Diéguez, M. Tetrahedron: Asymmetry 2014, 25, 258.
[32] Jiang, X.-B.; van den Berg, M.; Minnaard, A. J.; Feringa, B. L.; de Vries, J. G. Tetrahedron: Asymmetry 2004, 15, 2223.
[33] Panella, L.; Feringa, B. L.; de Vries, J. G.; Minnaard, A. J. Org. Lett. 2005, 7, 4177.
[34] Enthaler, S.; Erre, G.; Junge, K.; Michalik, D.; Spannenberg, A.; Marras, F.; Gladiali, S.; Beller, M. Tetrahedron: Asymmetry 2007, 18, 1288.
[35] Liu, Y.; Wang, Z.; Ding, K. Tetrahedron 2012, 68, 7581.
[36] Schmitz, C.; Leiter, W.; Franciò, G. Eur. J. Org. Chem. 2015, 2889.
[37] Ohta, T.; Miyake, T.; Seido, N.; Kumobayashi, H.; Takaya, H. J. Org. Chem. 1995, 60, 357.
[38] Kuroki, Y.; Asada, D.; Sakamaki, Y.; Iseki, K. Tetrahedron Lett. 2000, 41, 4603.
[39] Wu, S.; Wang, W.; Tang, W.; Lin, M.; Zhang, X. Org. Lett. 2002, 4, 4495.
[40] Qiu, L.; Wu, J.; Chan, S.; Au-Yeung, T. T.-L.; Ji, J.-X.; Guo, R.; Pai, C.-C.; Zhou, Z.; Li, X.; Fan, Q.-H.; Chan, A. S. C. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 5815.
[41] Le Gendre, P.; Braun, T.; Bruneau, C.; Dixneuf, P. H. J. Org. Chem. 1996, 61, 8453.
[42] Fehr, M. J.; Consiglio, G.; Scalone, M.; Schmid, R. J. Org. Chem. 1999, 64, 5768.
[43] Broger, E. A.; Burkart, W.; Hennig, M.; Scalone, M.; Schmid, R. Tetrahedron: Asymmetry 1998, 9, 4043.
[44] Jiang, Q; Xiao, D; Zhang, Z; Cao, P; Zhang, X. Angew. Chem., Int. Ed. 1999, 38, 516.
[45] Pignataro, L.; Bovio, C.; Civera, M.; Piarulli, U.; Gennari, C. Chem. Eur. J. 2012, 18, 10368.
[46] Burk, M. J.; Stammers, T. A.; Straub, J. A. Org. Lett. 1999, 1, 387.
[47] Liu, H.; Zhou, Y.-G.; Yu, Z.-K.; Xiao, W.-J.; Liu, S.-H.; He, H.-W. Tetrahedron 2006, 60, 11207.
[48] (a) Gridnev, I. D.; Higashi, N.; Imamoto, T. J. Am. Chem. Soc. 2001, 123, 4631.
(b). Gridnev, I. D.; Higashi, N.; Imamoto, T. Organometallics 2001, 20, 4542.
(c) Gridnev, I. D.; Yasutake, M.; Imamoto, T.; Beletskaya, I. P. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 5385.
(d) Imamoto, T.; Yashio, K.; Crépy, K. V. L.; Katagiri, K.; Takahashi, H.; Kouchi, M.; Gridnev, I. D. Organometallics 2006, 25, 908.
(e). Gridnev, I. D.; Imamoto, T.; Hoge, G.; Kouchi, M.; Takahashi, H. J. Am. Chem. Soc. 2008, 130, 2560.
(f). Tamura, K.; Sugiya, M.; Yoshida, K.; Yanagisawa, A.; Imamoto, T. Org. Lett. 2010, 12, 4400.
[49] (a) Rubio, M.; Suárez, A.; Álvarez, E.; Pizzano, A. Chem. Commun. 2005, 628.
(b) Rubio, M.; Vargas, S.; Suárez, A.; Álvarez, E.; Pizzano, A. Chem. Eur. J. 2007, 13, 1821.
[50] (a) Wassenaar, J.; Reek, J. N. H. J. Org. Chem. 2009, 74, 8403.
(b) Wassenaar, J.; Kuil, M.; Lutz, M.; Spek, A. L.; Reek, J. N. H. Chem. Eur. J. 2010, 16, 6509.
[51] (a) Wang, D.-Y.; Hu, X.-P.; Huang, J.-D.; Deng, J.; Yu, S.-B.; Duan, Z.-C.; Xu, X.-F.; Zheng, Z. Angew. Chem., Int. Ed. 2007, 46, 7810.
(b) Qiu, M.; Hu, X.-P.; Huang, J.-D.; Wang, D.-Y.; Deng, J.; Yu, S.-B.; Duan, Z.-C.; Zheng, Z. Adv. Synth. Catal. 2008, 350, 2683.
(c) Wang, D.-Y.; Huang, J.-D.; Hu, X.-P.; Deng, J.; Yu, S.-B.; Duan, Z.-C.; Zheng, Z. J. Org. Chem. 2008, 73, 2011.
[52] Fernández-Pérez, H.; Pericàs, M. A.; Vidal-Ferran, A. Adv. Synth. Catal. 2008, 350, 1984.
[53] (a) Vargas, S.; Suárez, A.; Álvarez, E.; Pizzano, A. Chem. Eur. J. 2008, 14, 9856.
(b) Chávez, M. Á.; Vargas, S.; Suárez, A.; Álvarez, E.; Pizzano, A. Adv. Synth. Catal. 2011, 353, 2775.
[54] Konno, T.; Shimizu, K.; Ogata, K.; Fukuzawa, S.-I. J. Org. Chem. 2012, 77, 3318.
[55] Sun, T.; Zhang, X. Adv. Synth. Catal. 2012, 354, 3211.
/
| 〈 |
|
〉 |