

Chinese Journal of Organic Chemistry >
Cu-Ag Bimetallic Nanoparticles Catalyzed Addition of Terminal Alkynes to Aldehydes
Received date: 2015-12-13
Revised date: 2016-01-15
Online published: 2016-02-01
Supported by
Project supported by the National Natural Science Foundation of China (No. 21172107), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
A copper-silver nanoparticles-catalyzed addition of terminal alkynes to aldehydes was developed for the synthesis of propargyl alcohols. With the promotion of bipyridine ligand, the bimetallic catalyst showed highly efficient to the reaction under neat condition, and good to excellent yields were achieved for various propargylic alcohols. In this catalytic reaction, a remarkable bimetallic synergistic effect was observed, in which Ag NPs worked as the co-catalyst and improved the catalytic activity of Cu NPs significantly. The nanoparticle catalyst could be recovered and reused effectively, and no obvious reduction on catalytic activity was observed after four recycles. Furthermore, a gram-scale reaction was also carried out successfully.
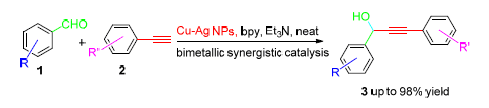
Wang Chao , Deng Nan , Wang Lingling , Xu Dingjian , Yao Xiaoquan . Cu-Ag Bimetallic Nanoparticles Catalyzed Addition of Terminal Alkynes to Aldehydes[J]. Chinese Journal of Organic Chemistry, 2016 , 36(5) : 1034 -1043 . DOI: 10.6023/cjoc201512018
[1] Chng, L. L.; Erathodiyi, N.; Ying, J. Y. Acc. Chem. Res. 2013, 46, 1825.
[2] For recent reviews, see: (a) Gao, G.; Moore, D.; Xie, R. G.; Pu, L. Org. Lett. 2002, 4, 4143.
(b) Pu, L.; Yu, H. B. Chem. Rev. 2001, 101, 757.
(c) Frantz, D. E.; Fässler, R.; Tomooka, C. S.; Carreira, E. M. Acc. Chem. Res. 2000, 33, 373.
(d) Srihari, P.; Singh, V. K.; Bhunia, D. C.; Yadav, J. S. Tertrahedron Lett. 2008, 49, 7132.
(e) Zheng, B.; Li, Z. Y.; Liu, F. P.; Wu, Y. H.; Shen, J.-J.; Bian, Q. H.; Hou, S. C.; Wang. M. Molecules 2013, 18, 15422.
[3] He, Q. W.; Ma. S. M. Chin. J. Org. Chem. 2002, 22, 375.
[4] (a) Usanov, D. L.; Yamamoto, H. J. Am. Chem. Soc. 2011, 133, 1286.
(b) Li, X. S.; Lu, G.; Kwok, W. H. J. Am. Chem. Soc. 2002, 124, 12636.
(c) Xu, M. H.; Pu, L. Org. Lett. 2002, 4, 4555.
(d) Moore, D.; Pu, L. Org. Lett. 2002, 4, 1855.
(e) Li, Z. B.; Pu, L. Org. Lett. 2004, 6, 1065.
(f) Xu, Z. Q.; Hen, C.; Xu, J. K.; Miao, M. B.; Yan, W. J.; Wang, R. Org. Lett. 2004, 6, 1193.
(g) Liu, L.; Wang, R.; Chen, C.; Xu, Z. Q.; Zhou, Y. F.; Ni, M.; Kang, Y. F.; Cai, H. Q.; Gong, M. Z. J. Org. Chem. 2004, 4095.
[5] Some reviews and examples, see: (a) Trost, B. M.; Krische, M. J. J. Am. Chem. Soc. 1999, 121, 6131.
(b) Tzalis, D.; Knochel, P. Angew. Chem., In. Ed. 1999, 38, 1463.
(c) Friedrich, K. In the Chemistry of Triple-boned Functional Groups, Supplement C, Eds.: Patai, S.; Rappoport, Z., John Wiley & Sons: New York, 1983, Chapter 28.
(d) Brandsma, L.; Verkruijsse, H. D. Synthesis of Acetylenes, Allenes and Cumulenes, Elsevier, Amsterdam, 1981.
(e) Liu, J.; An, Y.; Wang, Y. H.; Jiang, H. Y.; Zhang, Y. X.; Chen, Z. L. Chem. Eur. J. 2008, 14, 9131.
[6] (a) Frantz, D. E.; Fässler, R.; Tomooka, C. S.; Carreira, E. M. J. Am. Chem. Soc. 2000, 8, 1806.
(b) Boyall, D.; Frantz, D. E.; Carreira, E. M. Org. Lett. 2002, 4, 2605.
(c) Anand, N. K.; Carreira, E. M. J. Am. Chem. Soc. 2001, 123, 9687.
(d) Li, M.; Zhu, X. Z.; Yuan, K.; Cao, B. X.; Hou, X. L. Tetrahedron: Asymmentry. 2004, 15, 219.
(e) Xu, Z. Q.; Chen, C.; Xu, J. K.; Miao, M. B.; Yan, W. J.; Wang, R. Org. Lett. 2004, 6, 1193.
[7] (a) Takita, R.; Fukuta, Y.; Tsuji, R.; Ohshima, T.; Shibasaki, M. Org. Lett. 2005, 7, 1363.
(b) Takita, R.; Fukuta, Y.; Tsuji, R.; Ohshima, T.; Shibasaki, M. J. Am. Chem. Soc. 2005, 127, 13760.
[8] Wei, C. M.; Li, C. J. Green Chem. 2002, 4, 39.
[9] Dhondi, P. K.; Chisholm, J. D. Org. Lett. 2006, 8, 67.
[10] Ito, J.; Asai, R.; Nishiyama, H. Org. Lett. 2010, 12, 3860.
[11] (a) Asano, Y.; Ito, H.; Hara, K.; Aswamur, M. Organometallics 2008, 27, 5984.
(b) Asano, Y.; Hara, K.; Ito, H.; Aswamur, M. Org. Lett., 2007, 9, 3901.
[12] (a) Yao, X. Q.; Li, C. J. Org. Lett. 2005, 7, 4395.
(b) Yu, M.; Skouta, R.; Zhou, L.; Jiang, H.; Yao, X.; Li, C. J. J. Org. Chem. 2009, 74, 3378.
[13] Yao, X. Q.; Li, C. J. Org. Lett. 2006, 8, 1953.
[14] For selected reviews, see: (a) Yasukawa, T.; Miyamura, H.; Kobayashi, S. Chem. Soc. Rev. 2014, 43, 1450.
(b) Shaikhutdinov, S.; Freund, H.-J. Acc. Chem. Res. 2013, 46, 1673.
(c) Hervés, P.; Pérez Lorenzo, M.; Liz-Marzán, L. M.; Dzubiella, J.; Lu, Y.; Ballauff, M. Chem. Soc. Rev. 2012, 41, 5577.
(d) Dhakshinamoorthy, A.; Garcia, H. Chem. Soc. Rev. 2012, 41, 5262.
(e) Shiju, N. R.; Guliants, V. V. Appl. Catal. A: Gen. 2009, 356, 1.
(f) Somorjai, G. A.; Park, J. Y. Angew. Chem., Int. Ed. 2008, 47, 9212.
(g) Gu, Y.; Li, G. Adv. Synth. Catal. 2009, 351, 817.
[15] Yu, M.; Han, C. Y.; Wang, Y.; Zhu, L.; Yao, X. Chin. J. Org. Chem. 2013, 33, 492 (in Chinese).
(喻敏, 韩成燕, 王勇, 朱荔, 姚小泉, 有机化学, 2013, 33, 492.)
[16] (a) Yu, M.; Lin, M.; Han, C.; Li, Z.; Li, C. J.; Yao, X. Q. Tetrahedron Lett. 2010, 51, 6722.
(b) Han, C.; Yu, M.; Sun, W.; Yao, X. Q. Synlett 2011, 2363.
(c) Yu, M.; Wang, Y.; Sun, W.; Yao, X. Q. Adv. Synth. Catal. 2012, 354, 71.
(d) Sun, W.; Wang, Y.; Wu, X.; Yao, X. Q. Green Chem. 2013, 15, 2356.
(e) Xu, S.; Zeng, S.; Wang, Y.; Yu, M.; Zhu, L.; Yao, X. Q. Chin. J. Org. Chem. 2015, 35, 827 (in Chinese).
(徐森, 曾书伟, 王勇, 喻敏, 朱荔, 姚小泉, 有机化学, 2015, 35, 827.) (f) Lv, W.; Tian, J.; Deng, N.; Wang, Y.; Zhu, X.; Yao, X. Tetrahedron Lett. 2015, 56, 1312.
(g) Wang, Y.; Wu. C. L.; Nie, S. J.; Xu, D. J.; Yao, X. Q. Tetrahedron. Lett. 2015, 56, 6827.
[17] For selected reviews, see: (a) Sun, Z.; He, J. M.; Qu, M. N.; Li, K. S. Chin. J. Org. Chem. 2015, 35, 1250 (in Chinese).
(孙哲, 何金梅, 屈孟男, 李侃社, 有机化学, 2015, 35, 1250.) (b) Allen, A. E.; MacMillan, D. W. C. Chem. Sci. 2012, 3, 633.
(c) Zhou, J. Chem. Asian J. 2010, 5, 422.
(d) Su, Y. J.; Jia, W.; Jiao, N. Synthesis. 2011, 1678.
(e) Zhang, Y. L.; Yan, W. W.; Sun, Z. M.; Li, X. C.; Gao, J. P. RSCAdv. 2014, 4, 38040.
(f) Mohammad, R. N.; Yasamin, B.; Maryam, A. RSC. Adv. 2014, 4, 35844.
[18] (a) Wei, C. M.; Li, C. J. J. Am. Chem. Soc. 2002, 124, 5638.
(b) Wei, C. M.; Mague, J. T.; Li, C. J. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 5749.
[19] (a) Zhang, G.; Yi, H.; Zhang, G.; Deng, Y.; Bai, R.; Zhang, H.; Miller, J. T.; Kropf, A. J.; Bunel, E. E.; Lei, A. J. Am. Chem. Soc. 2014, 136, 924.
(b) Qi, X.; Li, Y.; Zhang, G.; Li, Y.; Lei, A.; Liu,; Lan, Y. Dalton Trans. 2015, 44, 11165.
(c) Yuan, J.; Wang, J.; Zhang, G.; Liu, C.; Qi, X.; Lan, Y.; Miller, J. T.; Kropf, J.; Bunel, E. E.; Lei, A. Chem. Commun. 2015, 51, 576.
(d) Tang, S.; Liu, Y.; Lei, A. Angew. Chem., Int. Ed. 2015, 54, 15850.
[20] Singh, M.; Sinha, I.; Singh, A. K.; Mandal, R. K. Colloid Surf., A 2005, 384, 668.
[21] Valodkar, M.; Modi, S.; Pal, A.; Thakore, S. Mater. Res. Bull. 2011, 46, 384.
[22] Tan, K. S.; Cheong, K. Y.; J. Nanopart. Res. 2013, 15, 1537.
[23] Li, Y. S.; Lu, Y. C.; Chou, K. S.; Liu, F. J. Mater. Res. Bull. 2010, 45, 1837.
[24] Grouchko, M.; Kamyshny, A.; Magdassi, S. J. Mater. Chem, 2009, 19, 3057.
[25] Butovsky, E.; Perelshtein, I.; Gedanken, A. J. Mater. Chem, 2012, 22, 15025.
[26] Kwok, H. N.; Penner, R. M. J. Electroanal. Chem. 2002, 522, 86.
/
| 〈 |
|
〉 |