

Chinese Journal of Organic Chemistry >
Nickel-Catalyzed Addition and Coupling Reaction of Aryl Triflates to Aldehydes
Received date: 2016-02-03
Revised date: 2016-03-09
Online published: 2016-03-18
Supported by
Project supported by the National Natural Science Foundation of China (No. 21372202) and the Program for New Century Excellent Talents in University (No. NCET-12-1086).
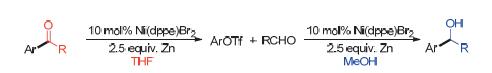
The nickel-catalyzed addition and coupling reaction between aryl triflates and aldehydes were developed. The reactions proceed smoothly in the presence of zinc powder with the use of 10 mol% Ni(dppe)Br2 as a catalyst. A range of aryl methanols and aryl ketones were isolated respectively in moderate to good yields in MeOH and THF solvent via direct addition or coupling reactions. Aliphatic and aromatic aldehydes were involved in this reaction, showing a broad substrate scope.
Zhang Mingdi , Chen Bin , Ge Chen , Liu Renrong , Gao Jianrong , Jia Yixia . Nickel-Catalyzed Addition and Coupling Reaction of Aryl Triflates to Aldehydes[J]. Chinese Journal of Organic Chemistry, 2016 , 36(7) : 1636 -1642 . DOI: 10.6023/cjoc201602007
[1] (a) Okude, Y.; Hirano, S.; Hiyama, T.; Nozaki, H. J. Am. Chem. Soc. 1977, 99, 3179.
(b) Jin, H.; Uenishi, J.; Christ, W. J.; Kishi, Y. J. Am. Chem. Soc. 1986, 108, 5644.
(c) Takai, K.; Tagashira, M.; Kuroda, T.; Oshima, K.; Utimoto, K.; Nozaki, H. J. Am. Chem. Soc. 1986, 108, 6048.
(d) Fürstner, A.; Shi, N. J. Am. Chem. Soc. 1996, 118, 2533.
(e) Fürstner, A.; Shi, N. J. Am. Chem. Soc. 1996, 118, 12349.
[2] (a) Quan, L.-G.; Lamrani, M.; Yamamoto, Y. J. Am. Chem. Soc. 2000, 122, 4827.
(b) Pletnev, A. A.; Larock, R. C. J. Org. Chem. 2002, 67, 9428.
(c) Solé, D.; Vallverdú, L.; Solans, X.; Font-Bardía, M.; Bonjoch, J. J. Am. Chem. Soc. 2003, 125, 1587.
(d) Cacchi, S.; Fabrizi, G.; Gavazza, F.; Goggiamani, A. Org. Lett. 2003, 289.
(e) Solé, D.; Serrano, O. Angew. Chem., Int. Ed. 2007, 46, 7270.
(f) Solé, D.; Serrano, O. J. Org. Chem. 2008, 73, 9372.
(g) Zhao, Y.-B.; Mariampillai, B.; Candito, D. A.; Laleu, B.; Li, M.; Lautens, M. Angew. Chem., Int. Ed. 2009, 48, 1849.
[3] (a) Majumdar, K. K.; Cheng, C.-H. Org. Lett. 2000, 2295.
(b) Rayabarapu, D. K.; Chang, H.-T.; Cheng, C.-H. Chem. Eur. J. 2004, 10, 2991.
(c) Hsieh, J. C.; Cheng, C.-H. Chem. Commun. 2005, 4554.
(d) Hu, J.-X.; Wu, H.; Li, C.-Y.; Sheng, W.-J.; Jia, Y.-X.; Gao, J.-R. Chem. Eur. J. 2011, 17, 5234.
(e) Yin, H.; Zhao, C.; You, H.; Lin, K.; Gong, H. Chem. Commun. 2012, 7034.
(f) Wu, F.; Lu, W.; Qian, Q.; Ren, Q.; Gong, H. Org. Lett. 2012, 14, 3044.
(g) He, J.-Q.; Chen, C.; Yu, W.-B.; Liu, R.-R.; Xu, M.; Li, Y.-J.; Gao, J.-R.; Jia, Y.-X. Tetrahedron Lett. 2014, 55, 2805.
(h) Zhao, C.; Jia, X.; Wang, X.; Gong, H. J. Am. Chem. Soc. 2014, 136, 17645.
[4] Correa, A.; Martin, R. J. Am. Chem. Soc. 2014, 136, 7253.
[5] Huang, Y.-C.; Majumdar, K. K.; Cheng, C.-H. J. Org. Chem. 2002, 67, 1682.
[6] (a) Kuriyama, M.; Shimazawa, R.; Enomoto, T.; Shirai, R. J. Org. Chem. 2008, 73, 6939.
(b) Infante, R.; Nieto, J.; Andrés, C. Org. Biomol. Chem. 2011, 9, 6691.
(c) Kuriyama, M.; Ishiyama, N.; Shimazawa, R.; Onomura, O. Tetrahedron 2010, 66, 6814.
(d) Yamamoto, T.; Ohta, T.; Ito, Y. Org. Lett. 2005, 7, 4153.
(e) DeBerardinis, A. M.; Turlington, M.; Pu, L. Org. Lett. 2008, 10, 2709.
(f) Yamamoto, T.; Furusawa, T.; Zhumagazin, A.; Yamakawa, T.; Oe, Y.; Ohta, T. Tetrahedron 2015, 71, 19.
(g) Hirose, T.; Sugawara, K.; Kodama, K. J. Org. Chem. 2011, 76, 5413.
(h) Majumdar, K. K.; Cheng, C.-H. Org. Lett. 2000, 2, 2295.
[7] (a) Rao, M. L. N.; Venkatesh, V. Banerjee, D. Tetrahedron 2007, 63, 12917.
(b) Silbestri, G. F.; Masson, R. B.; Lockhart, M. T.; Chopa, A. B. J. Organomet. Chem. 2006, 619, 1520.
(c) Andrus, M. B.; Ma, Y.; Zang, Y.; Songa, C. Tetrahedron Lett. 2002, 43, 9137.
(d) Ushijima, S.; Dohi, S.; Moriyama, K.; Togo, H. Tetrahedron 2012, 68, 1436.
(e) Baghos, V. B.; Doss, S. H.; Eskander, E. F. Org. Prep. Proced. Int. 1993, 25, 301.
(f) Meng, G.; Szostak, M. Org. Lett. 2015, 17, 4364.
(g) Huang, Y. C.; Majumdar, K. K.; Cheng, C.-H. J. Org. Chem. 2002, 67, 1682.
/
| 〈 |
|
〉 |