

Chinese Journal of Organic Chemistry >
Researches on the α-Aminoxylation between α-Azoleketones and 2,2,6,6-Tetramethylpiperidine-1-oxyl with Cu/O2
Received date: 2016-01-15
Revised date: 2016-03-16
Online published: 2016-04-05
Supported by
Project supported by the National Natural Science Foundation of China (No. 21272037), the Natural Science Foundation of Guangdong Province (No. S2013040014944) and the Guangdong University of Petrochemical Technology (No. 2015DCA037).
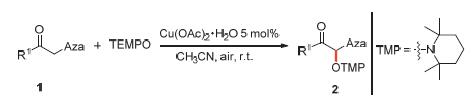
The α-aminoxylation between α-azoleketones and 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) was studied in this paper. Taking Cu(II) salts as catalyst and air as oxidant, a number of alkoxyamines have been synthesized via α-aminoxylation between α-azoleketones and TEMPO in good yields at room temperature. This strategy is highlighted by appealing features such as mild reaction condition, inexpensive catalyst, green oxidant and good yield.
Zhou Peng , Qiu Huihua , Pan Hongcheng , Shi Jicheng , Zhou Jianmin . Researches on the α-Aminoxylation between α-Azoleketones and 2,2,6,6-Tetramethylpiperidine-1-oxyl with Cu/O2[J]. Chinese Journal of Organic Chemistry, 2016 , 36(7) : 1596 -1601 . DOI: 10.6023/cjoc201601019
[1] (a) Erdmann, A.; Menon, Y.; Gros, C.; Molinier, N.; Novosad, N.; Samson, A.; Gregoire, J.; Long, C.; Ausseil, F.; Hlby, L.; Arimondo, P. B. Bioorg. Med. Chem. 2015, 23, 5946
(b) Lanier, M.; Sergienko, E.; Simão A. M.; Su, Y.; Chung, T.; Millán, J. L.; Cashman, J. R. Bioorg. Med. Chem. 2010, 18, 573
(c) Salerno, L; Modica, M. N.; Romeo, G.; Pittalà, V.; Siracusa, M. A.; Amato, M. E.; Acquaviva, R.; Giacomo, C. D.; Sorrenti, V. Eur. J. Med. Chem. 2012, 49, 118
(d) Peifer, C.; Bühler, S.; Hauser, D.; Kinkel, K.; Totzke, F.; Schächtele, C.; Laufer, S. Eur. J. Med. Chem. 2009, 44, 1788
(e) Wei, Q.-L.; Zhang, S.-S.; Gao, J.; Li, W.-H.; Xu, L.-Z.; Yu, Z.-G. Bioorg. Med. Chem. 2006, 14, 7146
(f) Pautus, S.; Sehr, P.; Lewis, J.; Fortuné, A.; Wolkerstorfer, A.; Szolar, O.; Guilligay, D.; Lunardi, T.; Décout, J.-L.; Cusack, S. J. Med. Chem. 2013, 56, 8915.
[2] (a) Hawker, C. J.; Bosmann, A. W.; Harth, E. Chem. Rev. 2001, 101, 3661.
(b) Benoit, D.; Chaplinski, V.; Braslau, R.; Hawker, C. J. J. Am. Chem. Soc. 1999, 121, 3904.
[3] (a) Sciannamea V.; Jérôme, R.; Detrembleur C. Chem. Rev. 2008, 108, 1104.
(b) Calabrese, D. R.; Ditter, D.; Liedel, C.; Blumfield, A.; Zentel, R.; Ober, C. K. ACS Macro. Lett. 2015, 4, 606.
[4] Wang, Z.-L.; An, X.-L.; Ge, L.-S.; Jin, J.-H.; Luo, X.; Deng, W.-P. Tetrahedron 2014, 70, 3788.
[5] (a) Dinca, E.; Hartmann, P.; Smr?ek, J.; Dix, I.; Jones, P. G.; Jahn, U. Eur. J. Org. Chem. 2012, 4461.
(b) Kirchberg, S.; Fröhlich, R.; Studer, A. Angew. Chem., Int. Ed. 2010, 49, 6877.
(c) Abeykoon, G. A.; Chatterjee, S.; Chen, J. S. Org. Lett. 2014, 16, 3248.
[6] Akagawa, K.; Fujiwara, T.; Sakamoto, S.; Kudo, K. Chem. Commun. 2010, 46, 8040.
[7] Bui, N.-N.; Ho, X.-H.; Mho, S.-I.; Jang, H.-Y. Eur. J. Org. Chem. 2009, 5309.
[8] Feng, P.; Song, S.; Zhang, L.-H.; Jiao, N. Synlett 2014, 25, 2717.
[9] Koike, T.; Yasu, Y.; Akita, M. Chem. Lett. 2012, 41, 999.
[10] Luo, X.; Wang, Z.-L.; Jin, J.-H.; An, X.-L.; Shen, Z.; Deng, W.-P. Tetrahedron 2014, 70, 8226.
[11] Li, Y.; Pouliot, M.; Vogler, T.; Renaud, P.; Studer, A. Org. Lett. 2012, 14, 4474.
[12] Xie, Y.-X.; Song, R.-J.; Liu, Y.; Liu, Y.-Y.; Xiang, J.-N.; Li, J.-H. Adv. Synth. Catal. 2013, 355, 3387.
[13] Selected reviews on reaction via Cu-catalyzed single electron transfer: (a) McCann, S. D.; Stahl, S. S. Acc. Chem. Res. 2015, 48, 1756.
(b) Yu, H.; Su, S.; Chi, Z.; Dang, Z. -M. Chin. J. Org. Chem. 2013, 33, 1628 (in Chinese). (于海珠, 苏圣钦, 张弛, 党智敏, 有机化学, 2013, 33, 1628.)
[14] Selected reviews on Cu-catalyzed oxidation with molecular oxygen: (a) Punniyamurthy, T.; Velusamy, S.; Iqbal J. Chem. Rev. 2005, 105, 2329.
(b) Campbell, A. N.; Stahl, S. S. Acc. Chem. Res. 2012, 45, 851.
(c) Allen, S. E.; Walvoord, R. R.; Padilla-Salinas, R.; Kozlowski, M. C. Chem. Rev. 2013, 113, 6234
Some examples on Cu-catalyzed oxidation with molecular oxygen: (d) Gao, H.; Wang, H.; Huang, Z.; Yao, L.; Peng, J.; Chen, C. Chin. J. Org. Chem. 2015, 35, 1707 (in Chinese). (高翯, 王瀚旸, 黄章杰, 姚丽萍, 彭进松, 陈春霞, 有机化学, 2015, 35, 1707.)
(e) Li, J.; Zhang, Z.; Li, C.; Luo, W.; Yang, S. Chin. J. Org. Chem. 2015, 35, 2199 (in Chinese). (李建晓, 张振明, 李春生, 罗维, 杨少容, 有机化学, 2015, 35, 2199.)
[15] (a) Tsai, A. S.; Wilson, R. M.; Harada, H.; Berqman, R. G.; Ellman, J. A. Chem. Commun. 2009, 3910
(b) Zhang, Y.; Zhang, Y.; Xiao, J.; Peng, Z.; Dong, W.; An, D. Eur. J. Org. Chem. 2015, 35, 7806.
[16] Kumar, D.; Reddy, V. B.; Kumar, A.; Mandal, D.; Tiwari, R.; Parang, K. Bioorg. Med. Chem. Lett. 2011, 21, 449.
/
| 〈 |
|
〉 |