

Chinese Journal of Organic Chemistry >
Preparation of Poly(vinyl chloride)-Based UV Absorbents and Their Resistence Property of Photoaging
Received date: 2015-12-31
Revised date: 2016-03-22
Online published: 2016-04-05
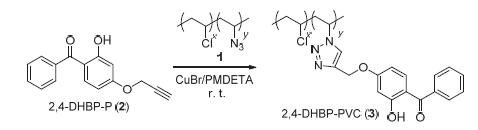
Poly(vinyl chloride) (PVC)-based light stabilizer was prepared by introducing the lower molecular UV absorbents into PVC using quantitative click reaction, which could avoid some defects (low permanence of light aging resistance property and environmental pollution) caused by poor polymer compatability of low molecular UV absorbent. Azide groups were firstly introduced into the backbone of PVC via a nucleophilic reaction to obtain PVC-N3 with different substitutive rate, and 2,4-dihydroxy-benzophenone (2,4-DHBP) was treated with propargyl bromide to prepare alkynyl-containing 2-hydroxy-4- (prop-2-ynyloxy)benzophenone (2,4-DHBP-P). Then, copper-catalyzed Husigen-Click cycloaddition reaction was performed between the pendant azide groups of PVC-N3 and alkynyl of 2,4-DHBP-P to afford the desired PVC-based UV absorbents (2,4-DHBP-PVC) with different amounts of benzophenone moieties. These 2,4-DHBP-PVC showed great resistance to photoaging while exposed to UV irradiation. Their ratio of carbonyl to methylene group were far lower than that of pure PVC (0.3331) after UV irradiating for 200 h, and the minimum value is about 0.03998.
Key words: poly(vinyl chloride); UV absorbents; photoaging
Wu Guojing, Zhu Chao, Weng Xiaodi, Sun Xiaodong, Lü Xuliang . Preparation of Poly(vinyl chloride)-Based UV Absorbents and Their Resistence Property of Photoaging[J]. Chinese Journal of Organic Chemistry, 2016 , 36(8) : 1963 -1969 . DOI: 10.6023/cjoc201512046
[1] Gesenhues, U. Polym. Degrad. Stab. 2000, 68, 185.
[2] Carlsson, J.; Krzymien, M.; Worsfold, J. J. Vinyl Addit. Technol. 1997, 3, 100.
[3] Naima, B. J. Vinyl Addit. Technol. 2002, 8, 45.
[4] Rabek, J. F. Polymer Photodegradation: Mechanisms and Experimental Methods, Chapman & Hall, London, UK, 1995. p.151
[5] Braun, D.; Richter, E.; Rabies, T. Angew. Makromol. Chem. 1999,271, 93.
[6] Magdy, W.; Emad, A. H.; Abir, S. Eur. Polym. J. 2005, 41, 2530.
[7] Pospisil, J.; Nespurek, S. Prog. Polym. Sci. 2000, 25, 1261.
[8] Allen, S. Polym. Photochem. 1983, 3, 167.
[9] Ormson, M.; Brown, G. Prog. React. Kinet. 1994, 19, 45.
[10] Gourrierec, D.; Ormson, M.; Brown, G. Prog. React. Kinet. 1994,19, 211.
[11] Kosower, M.; Huppert, D. Ann. Rev. Phys. Chem. 1986, 37, 127.
[12] Catalan, J.; Fabero, F.; Guijarro, S. J. Am. Chem. Soc. 1990, 112,747.
[13] Catalan, J.; Valle, C. J. Am. Chem. Soc. 1993, 115, 4321.
[14] Rieker, J.; Lemmert-Schmitt, E.; Goeller, G. J. Phys. Chem. 1992,96, 10225.
[15] Flom, R.; Barbara, F. Chem. Phys. Lett. 1983, 94, 488.
[16] Woessner, G.; Goeller, G.; Rieker, J. J. Phys. Chem. 1985, 89, 3629.
[17] Luston, J. Developments in Polymer Stabilisation-2, Applied Science Publishers, London, 1980, p. 185.
[18] Vink, P.; Scott, G. Development in Polymer Stabilization-3, Applied Science Publishers, London, 1980, p. 117.
[19] Billingham, N. C.; Calvert, P. D. Development in Polymer Stabilization-3, Applied Science Publishers, London, 1980, p. 139.
[20] Andrady, L.; Hamid, H.; Hu, X. J. Photochem. Photobiol. B 1998,46, 96.
[21] Binder, W. H.; Sachesenhofer, R. Macromol. Rapid Commun. 2007,28, 15.
[22] Wu, C. Y. Polym. Bull. 2006, 4, 76.
/
| 〈 |
|
〉 |