

Chinese Journal of Organic Chemistry >
Advances in Hydrogenation of Carboxylic Acid Derivatives and CO2 Using Triphos as the Coordination Ligand
Received date: 2016-03-08
Revised date: 2016-04-02
Online published: 2016-04-07
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21472215, 21572254).
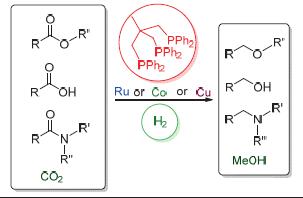
The reduction of carbon dioxide, carboxylic acids and their derivatives is one of the fundamental transformations both in academia and industry. Considering the increasing environmental issues, the use of molecular hydrogen as the reducing agent is especially attractive. Due to the mild reaction condition, high reactivity and easy modification of homogeneous catalysis, the development of highly efficient and selective homogeneous hydrogenation catalysts to achieve the goal is becoming a hot topic. Impressive progresses have been made using homogenous catalysts derived from transition metals and various ligands as catalysts. Among them, the catalytic system combined with a transition metal and CH3C(CH2PPh2)3 (triphos) usually shows unique reactivity and selectivity. This review will summarize the advance in the hydrogenation of carbon dioxide, carboxylic acids and their derivatives using Ru/triphos, Co/triphos and Cu/triphos as catalysts, as well as their reaction mechanisms.
Zhang Linli , Han Zhaobin , Zhang Lei , Li Mingxing , Ding Kuiling . Advances in Hydrogenation of Carboxylic Acid Derivatives and CO2 Using Triphos as the Coordination Ligand[J]. Chinese Journal of Organic Chemistry, 2016 , 36(8) : 1824 -1838 . DOI: 10.6023/cjoc201603014
[1] (a) Gunanathan, C.; Milstein, D. Chem. Rev. 2014, 114, 12024.
(b) Werkmeister, S.; Junge, K.; Beller, M. Org. Process Res. Dev. 2014, 18, 289.
(c) Pritchard, J.; Filonenko, G. A.; van Putten, R.; Hensen, E. J. M.; Pidko, E. A. Chem. Soc. Rev. 2015, 44, 3808.
[2] (a) Gribble, G. W. Chem. Soc. Rev. 1998, 27, 395.
(b) Seyden-Penne, J. Reductions by the Alumino- and Borohydrides in Organic Synthesis, 2nd ed.; Wiley, New York, 1997.
[3] (a) Noyori, R.; Ohkuma, T. Angew. Chem., Int. Ed. 2001, 40, 40.
(b) Blaser, H.-U.; Federsel, H.-J. Asymmetric Catalysis on Industrial Scale, 2nd ed., Weinheim, Wiley-VCH, 2010.
[4] McAlees, A. J.; McCrindle, R. J. Chem. Soc. C 1969, 2425.
[5] (a) Cokoja, M.; Bruckmeier, C.; Rieger, B.; Herrmann, W. A.; Kühn, F. E. Angew. Chem., Int. Ed. 2011, 50, 8510.
(b) Zhang, L.; Han, Z.; Zhao, X.; Wang, Z.; Ding, K. Angew. Chem., Int. Ed. 2015, 54, 6186.
[6] Wang, W. H.; Himeda, H.; Muckerman, J. T.; Manbeck, G. F.; Fujita, F. Chem. Rev. 2015, 115, 12936.
[7] Nishimura, S. Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis, Wiley, New York, 2001.
[8] Rieke, R.; Thakur, D.; Roberts, B.; White, G. J. Am. Oil Chem. 1997, 74, 333.
[9] Stein, M.; Breit, B. Angew. Chem., Int. Ed. 2013, 52, 2231.
[10] de Vries, J. G.; Elsevier, C. J. The Handbook of Homogeneous Hydrogenation, Wiley, Weinheim, 2007.
[11] Grey, R. A.; Pez, G. P.; Wallo, A. J. Am. Chem. Soc. 1981, 103, 7536.
[12] Matteoli, U.; Menchi, G.; Bianchi, M.; Piacenti, F. J. Mol. Catal. 1988, 44, 347.
[13] Teunissen, H. T.; Elsevier, C. J. Chem. Commun. 1997, 667.
[14] Hewertson, W.; Watson, H. R. J. Chem. Soc. 1962, 1490.
[15] van Engelen, M. C.; Teunissen, H. T.; de Vries, J. G.; Elsevier, C. J. J. Mol. Catal. A: Chem. 2003, 206, 185.
[16] (a) Bianchini, C.; Meli, A.; Peruzzini, M.; Vizza, F.; Zanobini, F. Coord. Chem. Rev. 1992, 120, 193.
(b) Hierso, J.-C.; Amardeil, R.; Bentabet, E.; Broussier, R.; Gautheron, B.; Meunier, P.; Kalck, P. Coord. Chem. Rev. 2003, 236, 143.
[17] Bianchini, C.; Meli, A.; Peruzzini, M.; Vizza, F.; Frediani, P.; Ramirez, J. A. Organometallics 1990, 9, 226.
[18] Barbaro, P.; Bianchini, C.; Meli, A.; Moreno, M.; Vizza, F. Organometallics 2002, 21, 1430.
[19] (a) Bianchini, C.; Meli, A.; Moneti, S.; Vizza, F. Organometallics 1998, 17, 2636.
(b) Bianchini, C.; Masi, D.; Meli, A.; Peruzzini, M.; Vizza, F.; Zanobini, F. Organometallics 1998, 17, 2495.
(c) Bianchini, C.; Meli, A.; Vizza, F. J. Organomet. Chem. 2004, 689, 4277.
[20] Barbaro, P.; Bianchini, C.; Frediani, P.; Meli, A.; Vizza, F. Inorg. Chem. 1992, 31, 1523.
[21] Mellone, I.; Bertini, F.; Gonsalvi, L.; Guerriero, A.; Peruzzini, M. Chimia 2015, 69, 331.
[22] (a) Dub, P. A.; Ikariya, T. ACS Catal. 2012, 2, 1718.
(b) Werkmeister, S.; Neumann, J.; Junge, K.; Beller, M. Chem. Eur. J. 2015, 21, 12226.
[23] Teunissen, H. T.; Elsevier, C. J. Chem. Commun. 1998, 1367.
[24] Berke, H. Book of Abstracts, XIIth FECHEM Conference on Organometallic Chemistry, Prague, 1997, PL 9.
[25] Rosato, D. V.; Rosato, M. V. Plastic Product Material and Process Selection Handbook, Elsevier, North Holland, 2004.
[26] Furst, M. R. L.; Goff, R. L.; Quinzler, D.; Mecking, S.; Botting C. H.; Cole-Hamilon D. J. Green Chem. 2012, 12, 472.
[27] vom Stein, T.; Meuresch, M.; Limper, D.; Schmitz, M.; Hölscher; Coetzee, J.; Cole-Hamilton, D. J.; Klankermayer, J.; Leitner, W. J. Am. Chem. Soc. 2014, 136, 13217.
[28] Wesselbaum, S.; vom Stein, T.; Klankermayer, J.; Leitner, W. Angew. Chem., Int. Ed. 2012, 51, 7499.
[29] Boardman, B.; Hanton, M. J.; van Rensburg, H.; Tooze, R. P. Chem. Commun. 2006, 2289.
[30] Hanton, M. J.; Tin, S.; Boardman, B. J.; Miller, P. J. Mol. Catal. A: Chem. 2011, 346, 70.
[31] Li, Y. H.; Topf, C.; Cui, X. J.; Junge, K.; Beller, M. Angew. Chem., Int. Ed. 2015, 54, 5196.
[32] Kilner, M.; Tyers, D. V.; Crabtree, S. P.; Wood, M. A. US 7709689, 2003 [Chem. Abstr. 2003, 139, 366612].
[33] Crabtree, S. P.; Tyers, D. V.; Sharif, M. WO 05/051907, 2005 [Chem. Abstr. 2005, 143, 43765].
[34] Rosi, L.; Frediani, M.; Frediani, P. J. Organomet. Chem. 2010, 695, 1314.
[35] Geilen, F. M. A.; Engendahl, B.; Harwardt, A.; Marquardt, W.; Klankermayer, J.; Leitner, W. Angew. Chem., Int. Ed. 2010, 49, 5510.
[36] Geilen, F. M. A.; Engendahl, B.; Hölscher, M.; Klankermayer, J.; Leitner, W. J. Am. Chem. Soc. 2011, 133, 14349.
[37] Phanopoulos, A.; White, A. J. P.; Long, N. J.; Miller, P. W. ACS Catal. 2015, 5, 2500.
[38] Cui, X. J.; Li, Y. H.; Topf, C.; Junge, K.; Beller, M. Angew. Chem., Int. Ed. 2015, 54, 10596.
[39] Constable, D. J. C.; Dunn, P. J.; Hayler, J. D.; Humphrey, G. R.; Leazer, J. L.; Linderman, R. J.; Lorenz, K.; Manley, J.; Pearlman, B. A.; Wells, A.; Zaks, A.; Zhang, T. Y. Green Chem. 2007, 9, 411.
[40] Magro, A. A. N.; Eastham, G. R.; Cole-Hamilton, D. J. Chem. Commun. 2007, 3154.
[41] Dodds, D. L.; Coetzee, J.; Klankermayer, J.; Brosinski, S.; Leitner, W.; Cole-Hamilton, D. J. Chem. Commun. 2012, 48, 12249.
[42] Coetzee, J.; Dodds, D. L.; Klankermayer, J.; Brosinski, S.; Leitner, W.; Slawin, A. M. Z.; Col-Hamilton, D. J. Chem. Eur. J. 2013, 19, 11039.
[43] Cabrero-Antonino, J. R.; Alberico, E.; Junge, K.; Junge, H.; Beller M. Chem. Sci. 2016, 7, 3432.
[44] Meuresch, M.; Westhues, S.; Leitner, W.; Klankermayer, J. Angew. Chem., Int. Ed. 2016, 55, 1392.
[45] Cabrero-Antonino, J. R.; Sorribes, I.; Junge, K.; Beller, M. Angew. Chem., Int. Ed. 2016, 55, 387.
[46] (a) Huff, C. A.; Sanford, M. S. J. Am. Chem. Soc. 2011, 133, 18122.
(b) Li, Y. N.; He, L. N.; Liu, A. H.; Lang, X. D.; Yang, Z. Z.; Yu, B.; Luan, C. R. Green Chem. 2013, 15, 2825.
(c) Khusnutdinova, J. R.; Garg, J. A.; Milstein, D. ACS Catal. 2015, 5, 2416.
(d) Kothandaraman, J.; Goeppert, A.; Czaun, M.; Olah, G. A.; Prakash, G. K. S. J. Am. Chem. Soc. 2016, 138, 778.
[47] Wesselbaum, S.; Moha, V.; Meuresch, M.; Brosinski, S.; Thenert, K. M.; Kothe, J.; vom Stein, T.; Englert, U.; Holscher, M.; Klankermayer, J.; Leitner, W. Chem. Sci. 2015, 6, 693.
[48] Beydoun, K.; vom Stein, T.; Klankermayer, J.; Leitner, W. Angew. Chem., Int. Ed. 2013, 52, 9554.
[49] Li, Y.; Sorribes, I.; Yan, T.; Junge, K.; Beller, M. Angew. Chem., Int. Ed. 2013, 52, 12156.
[50] Beydoun, K.; Ghattas, G.; Thenert, K.; Klankermayer, J.; Leitner, W. Angew. Chem., Int. Ed. 2014, 53, 11010.
[51] Beydoun, K.; Thenert, K.; Streng, E. S.; Brosinski, S.; Leitner, W.; Klankermayer, J. ChemCatChem 2016, 8, 135
[52] Li, Y.; Yan, T.; Junge, K.; Beller M. Angew. Chem., Int. Ed. 2014, 53, 10476.
[53] Savourey, S.; Lefevre, G.; Berthet, J.-C.; Cantat, T. Chem. Commun. 2014, 50, 14033.
[54] Sorribes, I.; Cabrero-Antonino, J. R.; Vicent, C.; Junge, K.; Beller, M. J. Am. Chem. Soc. 2015, 137, 13580.
[55] Korstanje, T. J.; van der Vlugt, J. I.; Elsevier, C. J.; de Bruin, B. Science 2015, 350, 298.
[56] Zall, C. M.; Linehan, J. C.; Appel A. M. ACS Catal. 2015, 5, 5301.
[57] Watari, R.; Kayaki, Y.; Hirano, S.; Matsumoto, N.; Ikariya, T. Adv. Synth. Catal. 2015, 357, 1369.
/
| 〈 |
|
〉 |