

Chinese Journal of Organic Chemistry >
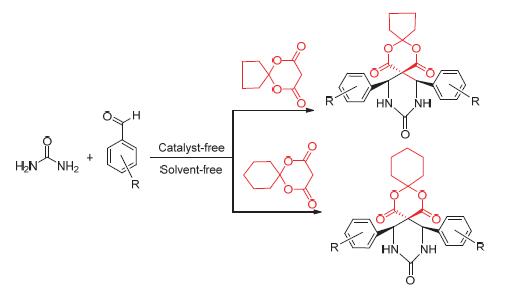
Efficient One-Pot Synthesis of Diphenyl-2,4-dioxa-8,10-diazaspiro-[5.5]undecane-1,5,9-trione Derivatives under Solvent-Free and Catalyst-Free
Received date: 2016-01-23
Revised date: 2016-03-22
Online published: 2016-04-07
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 20566004) and the Graduate Innovation Foundation of Jiangxi Province (No. YC2015-B023).
Twelve kinds of diphenyl-2,4-dioxa-8,10-diazaspiro[5.5]undecane-1,5,9-trione derivatives were synthesized by the three-component one-pot reaction of aromatic aldehydes with urea and 2,2-butylidene-1,3-dioxane-4,6-dione or 2,2-pentyli- dene-1,3-dioxane-4,6-dione under solvent-free and catalyst-free. The yields ranged from 49% to 63%. Its advantages were no solvent pollution and mild reaction conditions. Furthermore, a proposed reaction mechanism for this condensation reaction was speculated and the relationship between reaction speed and substituent groups was also described. All the compounds synthesized were confirmed by 1H NMR, 13C NMR, ESI-MS and IR techniques.
Xu Zhaohui , Zhou Peng , Lin Chunhua , Liu Deyong . Efficient One-Pot Synthesis of Diphenyl-2,4-dioxa-8,10-diazaspiro-[5.5]undecane-1,5,9-trione Derivatives under Solvent-Free and Catalyst-Free[J]. Chinese Journal of Organic Chemistry, 2016 , 36(8) : 1948 -1953 . DOI: 10.6023/cjoc201601032
[1] Arimoto, H.; Hayakawa, I.; Kuramoto, M.; Uemure, D. Tetrahedron Lett. 1998, 39, 861.
[2] Chou, T.; Kuramoto, M.; Otani, Y.; Shkano, M.; Yazawa, K.; Uemere, D. Tetrahedron Lett. 1996, 37, 3867.
[3] Rovnyak, G. C.; Kimball, S. D.; Barbara, B.; Gabriella, C.; John, D. D.; Jack, G.; Anders, H.; Mary, M.; James P. M. J. Med. Chem. 1995, 38, 119.
[4] Snider, B. B.; Shi, Z. J. Org. Chem. 1993, 58, 3828.
[5] Wessjohann, L. A.; Rivera, D. G.; Vercillo, O. E. Chem. Rev. 2009, 109, 796.
[6] Xiao, L. W.; Peng, X. X.; Zhou, Q. X.; Kou, W.; Shi, Y. R. Chin. J. Org. Chem. 2015, 35, 1204 (in Chinese).
(肖立伟, 彭晓霞, 周秋香, 寇伟, 时亚茹, 有机化学, 2015, 35, 1204.)
[7] Yang, K.; Xiang, J. B.; Bao, G. C.; Dang, Q.; Bai, X. ACS Comb. Sci. 2013, 15, 519.
[8] Dipak, P.; Debajyoti, B.; Mukut, G.; Hu, W. H. Mol. Divers. 2011, 15, 257.
[9] Srinivasa, R. J.; Divya, V.; Shubha, J. J. Chem. Pharm. Res. 2012, 4, 2373.
[10] Zhu, Y. L.; Huang, S. L.; Pan, Y. J. Eur. J. Org. Chem. 2005, 2354.
[11] Laishram, R. D.; Okram, M. S. Indian J. Chem. 2012, 51B, 1426.
[12] Anil, S.; Sanjay, K.; Jagir S. S. Indian J. Chem., 2004, 43B, 2482.
[13] Xu, Z. H.; Tu, Y. H. Chin. J. Org. Chem. 2015, 35, 1357 (in Chinese).
(许招会, 涂缘鸿, 有机化学, 2015, 35, 1357.)
[14] Naser, M.; Khalil, P.; Masoud, B.; Soudabeh, K. Asian J. Chem. 2013, 25, 3373.
[15] Wang, R.; Liu, Z. Q. J. Org. Chem. 2012, 77, 3952.
[16] Laurence, C.; Loïc, P.; Jérôme, M.; Vincent, C.; Xavier, M.; Christine, G. J. Org. Chem., 2015, 80, 595.
[17] Zeba, N. S.; Kulsum, K. ACS Sustainable Chem. Eng. 2014, 2, 1187.
[18] Wang, R.; Liu, Z. Q. J. Org. Chem. 2012, 77, 3952.
[19] Marcos, A. P. M.; Clarissa, P. F.; Dayse, N. M.; Lilian, B.; Pablo, M. Chem. Rev. 2009, 109, 4140.
[20] Maneeporn, P.; Romain, R.; Miho, H.; Waraporn, P.; Vudhichai, P.; Keiji, M. J. Org. Chem. 2015, 80, 6959.
[21] Haline, G. O. A.; Tatiani, B. L.; Aline, L. O.; Heibbe C. B. Oliveira.; Fabricio, M. S.; Fabio, C. G.; Roberto, Y. S.; Wender, A. Silva.; Brenno, A. D. N. J. Org. Chem. 2014, 79, 3383.
[22] Zheng, W. R.; Fu, Y.; Liu, L.; Guo, Q. X. Acta Phys.-Chim. Sin. 2007, 23, 1018 (in Chinese).
(郑文锐, 傅尧, 刘磊, 郭庆祥, 物理化学学报, 2007, 23, 1018.)
[23] Yan, N.; Xiong, B.; Liao, W. L.; Xu, Z. H. Chin. J. Org. Chem. 2010, 30, 1391 (in Chinese).
(严楠, 熊斌, 廖维林, 许招会, 有机化学, 2010, 30, 1391.)
/
| 〈 |
|
〉 |