

Chinese Journal of Organic Chemistry >
Copper-Catalyzed Coupling of Alkynes and Amines for the Synthesis of Propargyl Amines in the Presence of PhI(OAc)2
Received date: 2016-03-03
Revised date: 2016-04-11
Online published: 2016-04-20
In the presence of iodobenzene diacetate (PhI(OAc)2), a new CuBr catalyzed coupling reaction of alkynes and amines for the synthesis of propargyl amines was developed. When terminal alkynes, PhI(OAc)2, and amines were reacted in CH3CN at 70 ℃ for 3 h under N2 atmosphere and in the presence of CuBr, the desired propargyl amines were obtained in good yields. Furthermore, no matter aromatic or aliphatic alkynes, were all found to tolerate the reaction conditions. To the best of our knowledge, it is the first time that PhI(OAc)2 has been used for the synthesis of propargyl amines in one-pot operation from alkynes and amines.
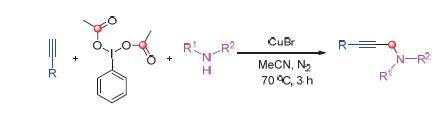
Key words: cuprous bromide; PhI(OAc)2; propargyl amines
Hu Dongyan , Li Mengsun . Copper-Catalyzed Coupling of Alkynes and Amines for the Synthesis of Propargyl Amines in the Presence of PhI(OAc)2 [J]. Chinese Journal of Organic Chemistry, 2016 , 36(8) : 1926 -1931 . DOI: 10.6023/cjoc201603003
[1] (a) Ohno, H.; Ohta, Y.; Oishi, S.; Fujii, N. Angew. Chem., Int. Ed. 2007, 46, 2295.
(b) Yan, B.; Liu, Y. Org. Lett. 2007, 9, 4323.
(c) Zhang, X.; Corma, A. Angew. Chem., Int. Ed. 2008, 47, 4358.
(d) Cao, K.; Zhang, F. M.; Tu, Y. Q.; Zhuo, X. T.; Fan, C. A. Chem. Eur. J. 2009, 15, 6332.
(e) Ohta, Y.; Oishi, S.; Fujii, N.; Ohno, H. Org. Lett. 2009, 11, 1979.
(f) Nakamura, H.; Onagi, S.; Kamakura, T. J. Org. Chem. 2005, 70, 2357.
(g) Sugiishi, T.; Kimura, A.; Nakamura, H. J. Am. Chem. Soc. 2010, 132, 5332.
(h) Nakamura, H.; Kamakura, T.; Ishikura, M.; Biellmann, J. F. J. Am. Chem. Soc. 2004, 126, 5958.
[2] (a) Farwick, A.; Helmchen, G. Org. Lett. 2010, 12, 1108.
(b) Jiang, B.; Xu, M. Angew. Chem., Int. Ed. 2004, 43, 2543.
(c) Yoon, T.; Shair, M. D.; Danishefsky, S. J.; Shulteo, G. K. J. Org. Chem. 1994, 59, 3752.
[3] (a) Giles, R. L.; Nkansah, R. A.; Looper, R. E. J. Org. Chem. 2010, 75, 261.
(b) Samai, S.; Nandi, G. C.; Singh, M. S. Tetrahedron Lett. 2010, 51, 5555.
(c) Nakamura, H.; Ishikura, M.; Sugiishi, T.; Kamakura, T.; Biellmann, J. F. Org. Biomol. Chem. 2008, 6, 1471.
(d) Trybulski, E. J.; Zhang, J.; Kramss, R. H.; Mangano, R. M. J. Med. Chem. 1993, 36, 3533.
[4] (a) Birkmayer, W.; Knol, J.; Riederer, P. J. Neural Transm. 1985, 64, 113.
(b) Chen, J. J.; Swope, D. M.; Dashtipour, K. Clin. Ther. 2007, 29, 1825.
[5] Murai, T.; Mutoh, Y.; Ohta, Y.; Murakami, M. J. Am. Chem. Soc. 2004, 126, 5968.
[6] Ahn, J. H.; Joung, M. J.; Yoon, N. M.; Oniciu, D. C.; Katritzky, A. R. J. Org. Chem. 1999, 64, 488.
[7] (a) Bieber, L. W.; da Silva, M. F. Tetrahedron Lett. 2004, 45, 8281.
(b) Fodor, A.; Kiss, A.; Debreczeni, N.; Hell, Z.; Gresits, I. Org. Biomol. Chem. 2010, 8, 4575.
(c) Wei, C.; Li, C. J. J. Am. Chem. Soc. 2002, 124, 5683.
(d) Shi, L.; Tu, Y. Q.; Wang, M.; Zhang, F. M.; Fan, C. A. Org. Lett. 2004, 6, 1001.
[8] Wei, C.; Li, Z.; Li, C. J. Org. Lett. 2003, 5, 4473.
[9] (a) Wei, C. M.; Li, C. J. J. Am. Chem. Soc. 2003, 125, 9584.
(b) Zhang, X.; Corma, A. Angew. Chem., Int. Ed. 2008, 47, 4358.
(c) Chng, L. L.; Yang, J.; Wei Y.; Ying, J. Y. Adv. Synth. Catal. 2009, 351, 2887.
[10] (a) Li, P. H.; Zhang, Y. C.; Wang, L. Chem. Eur. J. 2009, 15, 2045.
(b) Zeng, T. Q.; Chen, W. W.; Li, C. J. Green Chem. 2010, 12, 570.
[11] Ramu, E.; Varala, R.; Sreelatha, N.; Adapaa, S. R. Tetrahedron Lett. 2007, 48, 7184.
[12] (a) Zhang, Y.; Li, P.; Wang, M.; Wang, L. J. Org. Chem. 2009, 74, 4364.
(b) Yadav, J. S.; Subba Reddy, B. V.; Hara Gopal, A. V.; Patil, K. S. Tetrahedron Lett. 2009, 50, 3493.
[13] Chen, W. W.; Bi, H. P.; Li, C. J. Synlett 2010, 475.
[14] (a) Yu, D. Y.; Zhang, Y. G. Adv. Synth. Catal. 2011, 353, 163.
(b) Zeng, S. W.; Xu, S.; Wang, Y.; Yu, M.; Zhu, L.; Yao, X. Q. Chin. J. Org. Chem. 2015, 35, 827 (in Chinese).
(曾苏伟, 徐森, 姚小泉, 有机化学, 2015, 35, 827.)
[15] Chen, X. L.; Chen, T. Q.; Zhou, Y. B.; Au, C. T.; Han, L. B.; Yin, S. F. Org. Biomol. Chem. 2014, 12, 247.
[16] Tang, Y. C.; Xiao, T. B.; Zhou, L. Tetrahedron Lett. 2012, 53, 6199.
[17] Rahman, M.; Bagdi, A. K.; Majee, A.; Hajra, A. Tetrahedron Lett. 2011, 52, 4437.
[18] Berrichi, A.; Bachir, R.; Benabdallah, M.; Choukchou-Braham, N. Tetrahedron Lett. 2015, 56, 1302.
[19] Park, K.; Heo, Y.; Lee, S. Org. Lett. 2013, 15, 3322.
[20] Kabalka, G. W.; Venkataiah, B.; Dong, G. Tetrahedron Lett. 2004, 45, 729.
[21] Fodor, A.; Kiss, A.; Debreczeni, N.; Hell, Z.; Gresits, I. Org. Biomol. Chem. 2010, 8, 4575.
/
| 〈 |
|
〉 |