

Chinese Journal of Organic Chemistry >
Radical Promoted Difunctionalization of Unsaturated Carbon-Carbon Bonds in the Presence of Dioxygen
Received date: 2016-01-02
Revised date: 2016-01-20
Online published: 2016-04-29
Supported by
Project supported by the National Natural Science Foundation of China (No. 21202049), the Recruitment Program of Global Experts (1000 Talents Plan), the Fujian Hundred Talents Plan and the Program of Innovative Research Team of Huaqiao University (No. Z14X0047).
The difunctionalization of unsaturated carbon-carbon bonds is a powerful strategy for the synthesis of various organic compounds. Recently, the remarkable progress has been made in difunctionalization of unsaturated carbon-carbon bonds with dioxygen and radicals. The present protocol, which utilizes dioxygen as oxygen source, provides a green and atom economy approach to alcohols or ketones. This review will summarize the recent development in this area on the basis of different types of radicals.
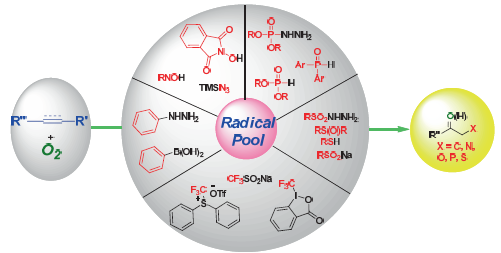
Key words: radical; difunctionalization; olefin; alkyne
Xu Jian , Song Qiuling . Radical Promoted Difunctionalization of Unsaturated Carbon-Carbon Bonds in the Presence of Dioxygen[J]. Chinese Journal of Organic Chemistry, 2016 , 36(6) : 1151 -1162 . DOI: 10.6023/cjoc201603042
[1] Kolb, H.-C.; VanNieuwenhze, M.-S.; Sharpless, K.-B. Chem. Rev. 1994, 94, 2483.
[2] Mai, W.-P.; Wang, J.-T.; Yang, L.-R.; Yuan, J.-W.; Mao, P.; Xiao, Y.-M.; Qu, L.-B. Chin. J. Org. Chem. 2014, 34, 1958 (in Chinese). (买文鹏, 王继涛, 杨亮茹, 袁金伟, 毛璞, 肖咏梅, 屈凌波, 有机化学, 2014, 349, 185.)
[3] (a) Nguyen, L.-M.; Diep, V.-V.; Phan, H.-T.; Niesor, E.-J.; Masson, D.; Guyon-Gellin, Y.; Buattini, E.; Severi, C.; Azoulay, R.; Bentzen, C.-L. WO 2004026245, 2004 [Chem. Abstr. 2004, 140, 287532].
(b) Erion, M.-D.; Jiang,-H.; Boyer, S.-H. US 20060046980, 2006 [Chem. Abstr. 2006, 144, 254238].
(c) Perumal, S.-K.; Adediran, S.-A.; Pratt, R.-F. Bioorg. Med. Chem. 2008, 16, 6987.
[4] (a) Ryglowski, A.; Kafarski, P. Tetrahedron 1996, 52, 10685.
(b) Kitamura, M.; Tokunaga, M.; Noyori, R. J. Am. Chem. Soc. 1995, 117, 2931.
[5] Maryanoff, B.-E.; Reitz, A.-B. Chem. Rev. 1989, 89, 863.
[6] Wei, W.; Ji, J.-X. Angew. Chem., Int. Ed. 2011, 50, 9097.
[7] Zhou, M.-X.; Zhou, Y.; Song, Q. Chem. Eur. J. 2015, 21, 10654.
[8] Chen, X.; Li, X.; Chen, X.; Qu, L.; Chen, J.; Sun, K.; Liu, Z.-D.; Bi, W.; Xia, Y.; Wu, H.; Zhao, Y.-F. Chem. Commun. 2015, 51, 3846.
[9] Zhang, P.-B.; Zhang, L.-L.; Gao, Y.-Z.; Xu, J.; Fang, H.; Tang, G.; Zhao, Y.-F. Chem. Commun. 2015, 51, 7839.
[10] Zhou, M.; Chen, M.; Zhou, Y.; Yang, K.; Su, J.; Du, J.; Song, Q. Org. Lett. 2015, 17, 1786.
[11] Yi, N.-N.; Wang, R.-J.; Zou, Hua.-X.; He, W.-B.; He, W. J. Org. Chem. 2015, 80, 5023.
[12] Zeng, Y.-F.; Tan, D.-H.; Lv, W.-X.; Li, Q.; Wang, H. Eur. J. Org. Chem. 2015, 4335.
[13] (a) Zhou, Y.; Rao, C.; Mai, S.; Song, Q. J. Org. Chem. 2016, 81, 2027.
(b) Zhou, Y.; Zhou, M.; Chen, M.; Su, J.; Du, J.; Song, Q. RSC Adv. 2015, 5, 103977.
[14] Taniguchi, T.; Idota, A.; Yokoyama, S.; Ishibashi, H. Tetrahedron Lett. 2011, 52, 4768.
[15] Denes, F.; Pichowicz, M.; Povie, G.; Renaud, P. Chem. Rev. 2014, 114, 2587.
[16] Lu, Q.; Zhang, J.; Zhao, G.; Qi, Y.; Wang, H.; Lei, A. J. Am. Chem. Soc. 2013, 135, 11481.
[17] Singh, A.-K.; Chawla, R.; Yadav, L. D. S. Tetrahedron Lett. 2014, 55, 2845.
[18] Wei, W.; Liu, C.-L.; Yang, D.-S.; Wen, J.-W.; You, J.-M.; Suo, Y.-R.; Wang, H.; Chem. Commun. 2013, 49, 10239.
[19] Wei, W.; Wen, J.-W.; Yang, D.-S.; Wu, M; You, J.-M.; Wang, H. Org. Biomol. Chem. 2014, 12, 7678.
[20] Liu, C.-R.; Ding, L.-H.; Guo, G.; Liu, W.-W. Eur. J. Org. Chem. 2016, 910.
[21] Singh, A.-K.; Chawla, R.; Yadav, L. D. S. Tetrahedron Lett. 2014, 55, 4742.
[22] Chawla, R.; Singh, A.-K.; Yadav, L. D. S. Eur. J. Org. Chem. 2014, 2032.
[23] Singh, A.-K.; Chawla, R.; Keshari, T.; Yadav, V.-K.; Yadav, L. D. S. Org. Biomol. Chem. 2014, 12, 8550.
[24] Keshari, T.; Yadav, V.-K.; Srivastava, V.-P.; Yadav, L. D. S. Green Chem. 2014, 16, 3986.
[25] Zhou, S.-F.; Pan, X.-Q.; Zhou, Z.-H.; Shoberu, A.; Zhang, P.-Z.; Zou, J.-P. J. Org. Chem. 2015, 80, 5348.
[26] Shi, X.-K.; Ren, X.-Y.; Ren, Z.-Y.; Li, J.; Wang, Y.-L.; Yang, S.-Z.; Gu, J.-X.; Gao, Q.; Huang, G.-S. Eur. J. Org. Chem. 2014, 5083.
[27] Lu, Q.; Zhang, J.; Wei, F.; Qi, Y.; Wang, H.; Liu, Z.; Lei, A. Angew. Chem., Int. Ed. 2013, 52, 7156.
[28] Taniguchi. T.; Idota, A.; Ishibashi, H. Org. Biomol. Chem. 2011, 9, 3151.
[29] Taniguchi, N. J. Org. Chem. 2015, 80, 7797.
[30] Kariya, A.; Yamaguchi, T.; Nobuta, T.; Tada, N.; Miura, T.; Itoh, A. RSC Adv. 2014, 4, 13191.
[31] Zhou, S.-F.; Pan, X.-Q.; Zhou, Z.-H.; Shoberu, A.; Zou, J.-P. J. Org. Chem. 2015, 80, 3682.
[32] Wang, H.-M.; Lu, Q.-Q.; Qian, C.-H.; Liu, C.; Liu, K.; Chen, K.; Lei, A. Angew. Chem., Int. Ed. 2016, 55, 1094.
[33] Liu, K.; Li, D.-P.; Zhou, S.-F.; Pan, X.-Q.; Shoberu, A.; Zou, J.-P. Tetrahedron 2015, 71, 4031.
[34] Wang, G.-Z.; He, X.-P.; Dai, J.-J.; Xu, H.-J. Chin. J. Org. Chem. 2014, 34, 837 (in Chinese). (王光祖, 赫侠平, 戴建军, 许华建, 有机化学, 2014, 34, 837.)
[35] Zhang, C.-P.; Wang, Z.-L.; Chen, Q.-Y.; Zhang, C.-T.; Gu, Y.-C.; Xiao, J.-C. Chem. Commun. 2011, 47, 6632.
[36] Deb, A.; Manna, S.; Modak, A.; Patra, T.; Maity, S.; Maiti, D. Agew. Chem., Int. Ed. 2013, 52, 9747.
[37] Maji, A.; Hazra, A.; Maiti, D. Org. Lett. 2014, 16, 4524.
[38] Lu, Q.; Liu, C.; Huang, Z.; Ma, Y.; Zhang, J.; Lei, A. Chem. Comun. 2014, 50, 14101.
[39] Yang, Y.; Liu, Y.-L.; Jiang, Y.; Zhang, Y.; Vicic, D.-A. J. Org. Chem. 2015, 80, 6639.
[40] Luo, H.-Q.; Zhang, Z.-P.; Dong, W.; Luo, X.-Z. Synlett 2014, 1307
[41] Wang, T.; Jiao, N. J. Am. Chem. Soc. 2013, 135, 11692.
[42] Sun, X.; Li, X.; Song, S.; Zhu, Y.; Liang, Y.-F.; Jiao, N. J. Am. Chem. Soc. 2015, 137, 6059.
[43] (a) Giglio, B. C.; Schmidt, V. A.; Alexanian, E. J. J. Am. Chem. Soc. 2011, 133, 13320.
(b) Schmidt, V. A.; Alexanian, E. J. Chem. Sci. 2012, 3, 1672.
[44] Bag, R.; Sar, D.; Punniyamurthy, T. Org. Lett. 2015, 17, 2010.
[45] Xia, X.-F.; Zhu, S.-L.; Gu, Z.; Wang, H.; Li, W.; Liu, X.; Liang, Y.-M. J. Org. Chem. 2015, 80, 5572.
[46] Lu, Q.; Liu, Z.; Luo, Y.; Zhang, G.; Huang, Z.; Wang, H.; Liu, C.; Miller, J.-T.; Lei, A. Org. Lett. 2015, 17, 3402.
[47] Dickschat, A.; Studer, A. Org. Lett. 2010, 12, 3972.
[48] Taniguchi, T.; Sugiura, Y.; Zaimoku, H.; Ishibashi, H. Angew. Chem., Int. Ed. 2010, 49, 10154.
[49] Taniguchi, T.; Zaimoku, H.; Ishibashi, H. Chem. Eur. J. 2011, 17, 4307.
[50] Kindt, S.; Jasch, H.; Heinrich, M.-R. Chem. Eur. J. 2014, 20, 6251.
[51] Su, Y.; Sun, X.; Wu, G.; Jiao, N. Angew. Chem., Int. Ed. 2013, 52, 9808.
/
| 〈 |
|
〉 |