

Chinese Journal of Organic Chemistry >
Research Progress in the Synthesis of 1,2,3-Triazole under Transition-Metal-Free
Received date: 2016-01-16
Revised date: 2016-04-02
Online published: 2016-05-03
Supported by
Project supported by the National Natural Science Foundation of China (No.21362008).
Because of its unique structure and chemical characteristics, 1,2,3-triazole was increasingly considered as an important structural motif in pharmaceuticals, agrochemicals, dyes and materials. Recently, main progress has been achieved under transition metal-catalysis. Considering the toxicity and potentially environmental pollution of transition metals, the novel, efficient and convenient synthetic methodologies of 1,2,3-triazole under transition metal-free conditions have been received widely attention. Based on our work and research interesting, the aim of this review is to give an overview of the progress on the diverse synthetic methodologies of 1,2,3-triazole with transition metal-free since 2010.
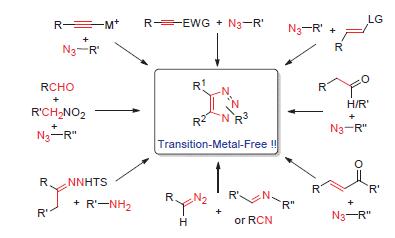
Key words: 1,2,3-triazole; transition metal-free; synthetic methods
Chen Yuxue , Zheng Chao , Peng Xiaochu , Fu Qingtan , Wu Luyong , Lin Qiang . Research Progress in the Synthesis of 1,2,3-Triazole under Transition-Metal-Free[J]. Chinese Journal of Organic Chemistry, 2016 , 36(8) : 1779 -1789 . DOI: 10.6023/cjoc201601020
[1] (a) Krivopalov, V. P.; Shkurko, O. P. Russ. Chem. Rev. 2005, 74, 339.
(b) Tome, A. C. In Science of Synthesis, Vol. 13, Eds.: Stor, R.; Gilchrist, T. Thieme, New York, 2004, p. 415.
(c) Jiang, Y.; Kuang, C. Proc. Chem. 2012, 24, 1983 (in Chinese).
(江玉波, 匡春香, 化学进展, 2012, 24, 1983.)
(d) Wang, J.; Li, L.; Zhang, G. Chin. J. Org. Chem. 2009, 29, 13 (in Chinese).
(王景梅, 李凌君, 张贵生, 有机化学, 2009, 29, 13.)
[2] Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596.
[3] Tornøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057.
[4] (a) Meldal; M.; Tornøe, C. W. Chem. Rev. 2008, 108, 2952.
(b) Boren, B. C.; Narayan, S.; Rasmussen, L. K.; Zhang, Li.; Zhao, H.; Lin, Z.; Jia, G.; Fokin, V. V. J. Am. Chem. Soc. 2008, 130, 8923.
(c) Barluenga, J.; Valdes, C.; Beltren, G.; Escribano, M.; Aznar, F. Angew. Chem., Int. Ed. 2006, 45, 6893.
[5] Baskin, J. M.; Prescher, J. A.; Laughlin, S. T.; Agard, N. J.; Chang, P. V.; Miller, I. A.; Lo, A.; Codelli, J. A.; Bertozzi, C. R. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 16793.
[6] Ning, X.; Guo, J.; Wolfert, M. A.; Boons, G.-J. Angew. Chem., Int. Ed. 2008, 47, 2253.
[7] Krasiński, A.; Fokin, V. V.; Sharpless, K. B. Org. Lett. 2004, 6, 1237.
[8] Kwok, S. W.; Fotsing, J. R.; Fraser, R. J.; Rodionov, V. O.; Fokin,V. V. Org. Lett. 2010, 12, 4217.
[9] Jiang, Y.; Kuang, C.; Yang, Q. Tetrahedron 2011, 67, 289.
[10] Meza-Aviña, M. E.; Patel, M. K.; Lee, C. B.; Dietz, T. J.; Croatt, M. P. Org. Lett. 2011, 13, 2984.
[11] Smith, C. D.; Greaney, M. F. Org. Lett. 2013, 15, 4826.
[12] Wu, L.; Chen, X.; Tang, M.; Song, X.; Chen, G.; Song, X.; Lin, Q. Synlett 2012, 23, 1529.
[13] Kloss, F.; Köhn, U.; Jahn, B. O.; Hager, M. D.; Görls, H.; Schubert, U. S. Chem. Asian J. 2011, 6, 2816.
[14] Li, J.; Zhang, Y.; Wang, D.; Wang, W.; Gao, T.; Wang, L.; Li, J.; Huang, G.; Chen, B. Synlett 2010, 1617.
[15] Cheng, G.; Zeng, X.; Shen, J.; Wang, X.; Cui, X. Angew. Chem., Int. Ed. 2013, 52, 13265.
[16] Zhang, H.; Tanimoto, H.; Morimoto, T.; Nishiyama, Y.; Kakiuchi, K. Org. Lett. 2013, 15, 5222.
[17] Quiclet-Sire, B.; Zard, S. Z. Synthesis 2005, 3319.
[18] Amantini, D.; Fringuelli, F.; Piermatti, O.; Pizzo, F.; Zunino, E.; Vaccaro, L. J. Org. Chem. 2005, 70, 6526.
[19] D'Ambrosio, G.; Fringuelli, F.; Pizzo, F.; Vaccaro, L. Green Chem. 2005, 7, 874.
[20] Wang, T.; Hu, X.-C.; Huang, X.-J.; Li, X.-S.; Xie, J.-W. J. Braz. Chem. Soc. 2012, 23, 1119.
[21] Quan, X.-J.; Ren, Z.-H.; Wang, Y.-Y.; Guan, Z.-H. Org. Lett. 2014, 16, 5728.
[22] Quan, X.-J.; Ren, Z.-H.; Wang, Y.-Y.; Guan, Z.-H. Org. Lett. 2015, 17, 393.
[23] Gao, Y.; Lam, Y. Org. Lett. 2006, 8, 3283.
[24] Dey, S.; Datta, D.; Pathak, T. Synlett 2011, 2521.
[25] Dey, S.; Pathak, T. RSC Adv. 2014, 4, 9275.
[26] Kayet, A.; Pathak, T. J. Org. Chem. 2013, 78, 9865.
[27] Kayet, A.; Dey, S.; Pathak, T. Tetrahedron Lett. 2015, 56, 5521.
[28] Bhaumik, A.; Samanta, S.; Pathak, T. J. Org. Chem. 2014, 79, 6895.
[29] Sahu, D.; Dey, S.; Pathak, T.; Ganguly, B. Org. Lett. 2014, 16, 2100.
[30] Alcaide, B.; Almendros, P.; Lázaro-Milla, C. Chem. Commun. 2015, 51, 6992.
[31] Li, W.; Wang, J. Angew. Chem., Int. Ed. 2014, 53, 14186.
[32] Li, W.; Du, Z.; Zhang, K.; Wang, J. Green Chem. 2015, 17, 781.
[33] Wan, J.-P.; Cao, S.; Liu. Y. J. Org. Chem. 2015, 80, 9028.
[34] Rogue, D. R.; Neill, J. L.; Antoon, J. W.; Stevens, E. P. Synthesis 2005, 2497.
[35] Hansen, S. G.; Jensen, H. H. Synlett 2009, 3275.
[36] Wu, L.; Chen, Y.; Luo, J.; Sun, Q.; Peng, M.; Lin, Q. Tetrahedron Lett. 2014, 55, 3847.
[37] Augustine, J. K.; Boodappa, C.; Venkatachaliah, S. Org. Biomol. Chem. 2014, 12, 2280.
[38] Penthala, N. R.; Madadi, N. R.; Janganati, V.; Crooks, P. A. Tetrahedron Lett. 2014, 55, 5562.
[39] Ramachary, D. B.; Ramakumar, K.; Narayana, V. V. Chem. Eur. J. 2008, 14, 9143.
[40] Ramachary, D. B.; Shashank, A. B. Chem. Eur. J. 2013, 19, 13175.
[41] Danence, L. J. T.; Gao, Y.; Li, M.; Huang, Y.; Wang, J. Chem. Eur. J. 2011, 17, 3584.
[42] Krishna, P. M.; Ramachary, D. B.; Peesapatia, S. RSC Adv. 2015, 62062.
[43] Belkheira, M.; Abed, D. E.; Pons, J.-M.; Bressy, C. Chem. Eur. J. 2011, 17, 12917.
[44] Wang, L.; Peng, S.; Danence, L. J. T.; Gao, Y.; Wang, J. Chem. Eur. J. 2012, 18, 6088.
[45] Li, W.; Jia, Q.; Du, Z.; Wang, J. Chem. Commum. 2013, 49, 10187.
[46] Ramachary, D. B.; Shashank, A. B.; Karthik, S. Angew. Chem., Int. Ed. 2014, 53, 10420.
[47] Shashank, A. B.; Karthik, S.; Madhavachary, R.; Ramachary, D. B. Chem. Eur. J. 2014, 20, 16877.
[48] Ramachary, D. B.; Krishna, P. M.; Gujral, J.; Reddy, G. S. Chem. Eur. J. 2015, 21, 16775.
[49] Jia, Q.; Yang, G.; Chen, L.; Du, Z.; Wei, J.; Zhong, Y.; Wang, J. Eur. J. Org. Chem. 2015, 3435.
[50] Sengupta, S.; Duan, H.; Lu, W.; Petersen, J. L.; Shi, X. Org. Lett. 2008, 10, 1493.
[51] Ponpandian, T.; Muthusubramanian, S. Tetrahedron Lett. 2012, 53, 59.
[52] Ali, A.; Corrêa, A. G.; Alves, D.; Zukerman-Schpector, J.; Westermann, B.; Ferreiraa, M. A. B.; Paixão, M. W. Chem. Commun. 2014, 50, 11926.
[53] Chai, H.; Guo, R.; Yin, W.; Cheng, L.; Liu, R.; Chu, C. ACS Comb. Sci. 2015, 17, 147.
[54] Maurya, R. A.; Adiyala, P. R.; Chandrasekhar, D.; Reddy, C. N.; Kapure, J. S.; Kamal, A. ACS Comb. Sci. 2014, 16, 466.
[55] John, J.; Thomas, J.; Parekh, N.; Dehaen, W. Eur. J. Org. Chem. 2015, 4922.
[56] Thomas, J.; John, J.; Parekh, N.; Dehaen, W. Angew. Chem., Int. Ed. 2014, 53, 10155.
[57] Wu, L.; Wang, X.; Chen, Y.; Huang, Q.; Lin, Q.; Wu, M. Synlett 2016, 27, 437.
[58] González-Calderón, D.; Santillán-Iniesta, I.; González-González, C. A.; Fuentes-Benítes, A.; González-Romero, C. Tetrahedron Lett. 2015, 56, 514.
[59] González-Calderón, D.; Paz, J. G. A.-D.; González-González, C. A.; Fuentes-Benítes, A.; González-Romero, C. Tetrahedron Lett. 2015, 56, 1713.
[60] Sakai, K.; Hida, N.; Kondo, K. Bull. Chem. Soc. Jpn. 1986, 59, 179.
[61] van Berkel, S. S.; Brauch, S.; Gabriel, L.; Henze, M.; Stark, S.; Vasilev, D.; Wessjohann, L. A.; Abbas, M.; Westermann, B. Angew. Chem., Int. Ed. 2012, 51, 5343.
[62] Cai, Z.-J.; Lu, X.-M.; Zi, Y.; Yang, C.; Shen, L.-J.; Li, J.; Wang S.-Y.; Ji, S.-J. Org. Lett. 2014, 16, 5108.
[63] Chen, Z.; Yan, Q.; Liu, Z.; Zhang, Y. Chem. Eur. J. 2014, 20, 17635.
[64] Bai, H.-W.; Cai, Z.-J.; Wang, S.-Y.; Ji S.-J. Org. Lett. 2015, 17, 2898.
[65] Chen, J.-H.; Liu, S.-R.; Chen, K. Chem. Asian J. 2010, 5, 328.
[66] Mani, N. S.; Fitzgerald A. E. J. Org. Chem. 2014, 79, 8889.
[67] He, Y.; Sun, E.; Zhao, Y.; Hai, L.; Wu, Y. Tetrahedron Lett. 2014, 55, 111.
[68] Kazancioglu, E. A.; Kazancioglu, M. Z.; Fistikci, M.; Secen, H.; Altundas, R. Org. Lett. 2013, 15, 4790.
[69] Loren, J. C.; Sharpless, K. B. Synthesis 2005, 1514.
/
| 〈 |
|
〉 |