

Chinese Journal of Organic Chemistry >
Synthesis, Antitumor and Anti-inflammatory Activities of Isolongifolanonyl Pyrazole Derivatives
Received date: 2016-03-14
Revised date: 2016-04-11
Online published: 2016-05-06
Supported by
Project supported by the University Science Research Project of Jiangsu Province (No.14KJ220001), the Natural National Science Foundation of China (No.31470529), and the Open Funding of Jiangsu Key Laboratory of Biomass Energy and Materials (No.JSBEM2014010).
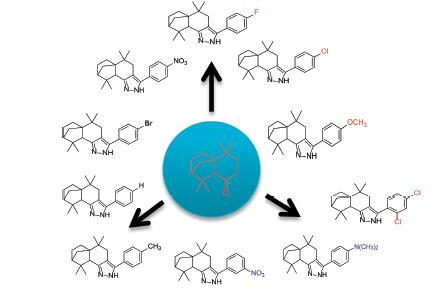
A series of new isolongifolanonyl pyrazole derivatives 3a~3j were synthesized by aldol-condensation, cyclization and dehydroaromatization from isolongifolanone. The yields of products ranged from 69.5% to 76.5%. The chemical structures of compounds obtained were elucidated by 1H NMR, 13C NMR, IR, MS spectra and elemental analysis. The strucure of compound 3i was determined by X-ray single crystal diffraction. Their inhibitory activities against human hepatoma cell (HepG2) and human umbilical vein endothelial cell (HUVECs) were evaluated. The results indicated that compounds 3h and 3i[IC50=(15.42±0.6), (4.74±0.3) μmol/L] showed obvious inhibitory activity against HUVECs. Compounds 3a, 3c,3e and 3j[IC50=(5.27±0.5), (6.71±0.4), (4.68±0.2), (4.57±0.5)] μmol/L showed better antitumor activity against HepG2.
Rui Jian , Yang Jianlai , Huang Jianfeng , Wang Jiayu , Xu Xua , Xu Haijun , Wang Shifa . Synthesis, Antitumor and Anti-inflammatory Activities of Isolongifolanonyl Pyrazole Derivatives[J]. Chinese Journal of Organic Chemistry, 2016 , 36(9) : 2183 -2190 . DOI: 10.6023/cjoc201603023
[1] Krishnaiah, A.; Narsaiah, B. J. Fluorine Chem. 2002, 115, 9.
[2] Du, H. T.; Du, H. J.; Ouyang, G. P. Chin. J. Org. Chem. 2008, 28, 356(in Chinese). (杜海堂, 杜海军, 欧阳贵平, 有机化学, 2008, 28, 356.)
[3] Tong, J. Y.; Shi, Y. X.; Liu, X. H.; Sun, N. B.; Li, B. J. Chin. J. Org. Chem. 2012, 32, 2373(in Chinese). (童建颖, 石延霞, 刘幸海, 孙娜波, 李宝聚, 有机化学, 2012, 32, 2373.)
[4] Nasr, T.; Bondock, S.; Eid, S. Eur. J. Med. Chem. 2014, 84, 492.
[5] Fadda, A. A.; El-Mekawy, R. E.; El-Shafei, A. I. J. Porphyrins Phthalocyanines 2015, 19, 754.
[6] Palanisamy, P.; Kumaresan, S. RSC Adv. 2013, 3, 4705.
[7] Gökhan-Kelekçi, N.; Yabano?lu, S.; Küpeli, E. Bioorg. Med. Chem. 2007, 15, 5775.
[8] Lv, P. C.; Li, H. Q.; Sun, J.; Zhou, Y.; Zhu, H. L. Bioorg. Med. Chem. 2010, 18, 4606.
[9] Goda, F. E.; Maarouf, A. R.; El-Bendary, E. R. Saudi Pharm. J. 2003, 11, 111.
[10] Rüger, A. J.; Nieger, M.; Bräse, S. Tetrahedron 2012, 68, 8824.
[11] Dai, H.; Chen, J.; Li, H.; Dai, B. J.; He, H. B.; Fang, Y.; Shi, Y. J. Molecules 2016, 21, 276.
[12] Foster, R. S.; Jakobi, H.; Harrity, J. P. A. Org. Lett. 2012, 14, 4859.
[13] Kawamori, T.; Rao, C. V.; Seibert, K.; Reddy, B. S. Cancer Res. 1998, 58, 409.
[14] Hou, Q. Q.; Li, Z. N.; Liu, C. L. Pesticides 2002, 41, 41(in Chinese). (侯春青, 李志念, 刘长令, 农药, 2002, 41, 41.)
[15] Kubota, M.; Yamamoto, K. JP 10130267, 1998[Chem. Abstr. 1998, 129, 41126.]
[16] Gordien, A. Y.; Gray, A. I.; Franzblau, S. G.; Seidel, V. J. Eth-nopharmacol. 2009, 126, 500.
[17] Bichlmaier, I.; Kurkela, M.; Siiskonen, A.; Finel, M.; Kauhaluoma, J. Y. Bioorg. Med. Chem. 2007, 35, 386.
[18] Zhang, A. J.; Klun, J. A.; Wang, S. F. J. Med. Entomol. 2009, 46, 100.
[19] Zhang, A.; Carroll, J.; Wang, S.; Klun, J. A. WO 200901209, 2009[Chem. Abstr. 2009, 150, 144686.]
[20] El-Sayed, M. A.; Abdel-Aziz, N. I.; Abdel-Aziz, A. A.; El-Azab, A. S.; ElTahir, K. E. H. Bioorg. Med. Chem. 2012, 20, 3306.
[21] Thurmond, R. L.; Beavers, M. P.; Cai, H.; Meduna, S. P.; Gustin, D. J.; Sun, S.; Almond. H. J.; Karlsson, L.; Edwards, J. P. J. Med. Chem. 2004, 47,4799.
[22] Dinges, J.; Albert, D. H.; Arnold, L. D.; Ashworth, K. L.; Akritopoulou-Zanze, I.; Bousquet, P. F.; Bouska, J. J.; Cunha, G. A.; Davidsen, S. K.; Diaz, G. J.; Djuric, S. W.; Gasiecki, A. F.; Gintant, G. A.; Gracias, V. J.; Harris, C. M.; Houseman, K. A.; Huthins, C. W.; Johnson, E. F.; Li, H.; Marcotte, P. A.; Martin, R. L.; Michaelids, M. R.; Nyein, M.; Sowin, T. J.; Su, Z.; Tapang, P. H.; Xia, Z.; Zhang, H. Q. J. Med. Chem. 2007, 50, 2011.
[23] Klein, O.; Aguilar-Parrilla, F.; Lopez, J. M.; Jagerovic, N.; Elguero, J.; Limbach, H. H. J. Am. Chem. Soc. 2004, 126, 11718.
/
| 〈 |
|
〉 |