

Chinese Journal of Organic Chemistry >
Design and Synthesis of Aromatic Amide Foldamer Based on Anthradiamine and Isophthalic Acid Derivatives
Received date: 2016-04-24
Revised date: 2016-05-20
Online published: 2016-05-24
Supported by
Project supported by the Science and Technology Commission of Shanghai Municipality (No. 13M1400200), the National Natural Science Foundation of China (Nos. 21102017, 21472023).
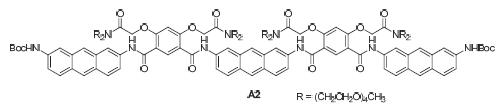
A kind of fluorescence aromatic amide oligomer which based on 2,7-anthradiamine and 4,6-dihydroxyisophthalic acid derivatives is designed and synthesized. Tetraoxatridecanyl groups were introduced into the oligomers to improve their solubility in polar solvent. These structures were characterized by 1H NMR and HRMS. Fluorescence titration experiments indicated that A2 recognized 1,3,5-benzene-tricarboxylate ion by host-guest interaction. The apparent binding constant of the complex was measured to be about 1.95×103 L·mol-1, which opened a door on explore the supramolecular host-guest system by the track of fluorescence.
Key words: aromatic amide; fluorescence; foldamer
Wang Yi , Wang Hui , Zhang Dan-Wei , Li Zhan-Ting . Design and Synthesis of Aromatic Amide Foldamer Based on Anthradiamine and Isophthalic Acid Derivatives[J]. Chinese Journal of Organic Chemistry, 2016 , 36(7) : 1580 -1585 . DOI: 10.6023/cjoc201604049
[1] Gellman, S. H. Acc. Chem. Res. 1998, 31, 173.
[2] Li, X.; Wu, Y.-D.; Yang, D. Acc. Chem. Res. 2008, 41, 1428.
[3] Huc, I. Eur. J. Org. Chem. 2004, 17.
[4] Gong, B. Acc. Chem. Res. 2008, 41, 1376.
[5] Saraogi, I.; Hamilton, A. D. Chem. Soc. Rev. 2009, 38, 1726.
[6] Zhang, D.-W.; Zhao, X.; Hou, J.-L.; Li, Z.-T. Chem. Rev. 2012, 112, 5271.
[7] Juwarker, H.; Jeong, K.-S. Chem. Soc. Rev. 2010, 39, 3664.
[8] Hua, Y.; H. Flood, A. H. Chem. Soc. Rev. 2010, 39, 1262.
[9] Zhang, C.; Peng, X.-X.; Chen, C.-F. Chin. J. Chem. 2011, 29, 2606.
[10] Wu, X. X.; Liang, G. X.; Ji, G.; Fun, H.-K.; He, L.; Gong, B. Chem. Commun. 2012, 48, 2228.
[11] Selve, C.; Ravey, J. C.; Stebe, M. J.; El Moudjahid, C.; Moumni, E. M.; Delpuech, J. I. Tetrahedron 1991, 47, 411.
[12] Xu, Y.-X.; Wang, G.-T.; Zhao, X.; Jiang, X.-K.; Li, Z.-T. J. Org. Chem. 2009, 74, 7267.
[13] Hosseini, M.; Ghafarloo, A.; Ganjali, M. R.; Faridbod, F.; Norouzi, P.; Niasari, M. S. Sens. Actuators B 2014, 198, 411.
[14] Zhu, M.; Yuan, M.; Liu, X. ; Xu, J.; Lv, J.; Huang, C. Org. Lett. 2008, 10, 1481.
[15] Benesi, H. A.; Hildebrand. J. H. J. Am. Chem. Soc. 1949, 71, 2703.
[16] Barra, M.; Bohne, C.; Scaiano. J. C. J. Am. Chem. Soc. 1990, 112, 8075.
[17] Farbeind, I. G. DE 497503, 1925 [Chem. Abstr. 1931, 25, 4445].
[18] Adrian, C.-Z.; Luis, A. M.; Eduardo R.-B.; Armando, G.-L. Synth. Commun. 1998, 28, 3461.
/
| 〈 |
|
〉 |