

Chinese Journal of Organic Chemistry >
Synthesis and Properties of Benzocyclobutene-Functionalized Thermosetting Polycarbonate
Received date: 2016-04-07
Revised date: 2016-05-30
Online published: 2016-06-02
Supported by
Project supported by the Natural Science Foundation of China (NSFC, No.21374131, 21574146 and 21504103) and the Science and Technology Commission of Shanghai Municipality (15ZR1449200).
A novel benzocyclobutene (BCB)-functionalized polycarbonate was synthesized. This polymer showed good solu-bility in organic solvents and could be processed by solution method. After cured at high temperature, the polymer exhibited high thermostability with 5% weight loss temperature of 451℃ and a char yield of 33% at 1000℃ in nitrogen. The cured polymer showed good hydrophobicity with water uptake of 0.21% after immersion in boiling water for 24 h. Moreover, a copolymer and poly(bisphenol A carbonate) were synthesized by bisphenol A monomer to study the influence of the existence of BCB groups on the properties of the aromatic polycarbonate. The results of comparative study showed that BCB groups can effectively improve the thermostability and hydrophoblicity of aromatic polycarbonates.
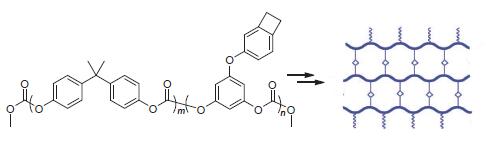
Key words: polycarbonate; benzocyclobutene; synthesis; thermosetting polymer
Li Zhiyong , Cao Weiguo , Sun Jing , Fang Qiang . Synthesis and Properties of Benzocyclobutene-Functionalized Thermosetting Polycarbonate[J]. Chinese Journal of Organic Chemistry, 2016 , 36(10) : 2442 -2448 . DOI: 10.6023/cjoc201604013
[1] Bendler, J. T.; Boyles, D. A.; Edmondson, C. A.; Filipova, T.; Fontanella, J. J.; Westgate, M. A.; Wintersgill, M. C. Macromolecules 2013, 46, 4024.
[2] Yoonessi, M.; Gaier, J. R. ACS Nano 2010, 4, 7211.
[3] Tamarit-López, J.; Morais, S.; Bañuls, M.; Puchades, R.; Ma-quieira, Á. Anal. Chem. 2010, 82, 1954.
[4] Zhou, J.; Lubineau, G. ACS Appl. Mater. Interfaces 2013, 5, 6189.
[5] Méndez-Liñán, L.; López-Garzón, F. J.; Domingo-García, M.; Pérez-Mendoza, M. Energy Fuels 2010, 24, 3394.
[6] Moon, S. I.; Extrand, C. W. Ind. Eng. Chem. Res. 2009, 48, 8961.
[7] Lyu, M.-Y.; Lee, J. S.; Pae, Y. J. Appl. Polym. Sci. 2001, 80, 1814.
[8] Baick, I. H.; Yang, W. J.; Ahn, Y. G.; Song, K. H.; Choi, K. Y. J. Appl. Polym. Sci. 2015, 132, 41609.
[9] Awwad, S. H.; Filipova, T. S.; Boyles, D. A. Polym. Prepr. 2011, 52(1), 368.
[10] Laughner, M. K.; Farah, H. WO 9215643, 1992[Chem. Abdtr. 1993, 118, 256003].
[11] Boyles, D. A.; Filipova, T. S.; Bendler, J. T.; Longbrake, G.; Reams, J. Macromolecules 2005, 38, 3622.
[12] Filipova, T. S.; Boyles, D. A.; Schroeder, M. J.; Fontanella, J. J.; Wintersgill, M. C.; Edmondson, C. A.; Bendler, J. T. Polym. Prepr. 2009, 50(2), 543.
[13] Delassus, S. L.; Howell, B. A. Macromolecules 1994, 27, 1307.
[14] Tian, S.; Sun, J.; Jin, K.; He, F.; Zheng, S.; Fang, Q. ACS Appl. Mater. Interfaces 2014, 6, 20437.
[15] Hahn, S. F.; Martin, S. J.; Mckelvy, M. L.; Patrick, D. W. Macro molecules 1993, 26, 3870.
[16] Marks, M. J.; Erskine, J. S.; McCrery, D. A. Macromolecules 1994, 27, 4114.
[17] Wang, J.; Piskun, I.; Craig, S. L. ACS Macro Lett. 2015, 4, 834.
[18] Cheng, Y.; Yang, J.; Jin, Y.; Deng, D.; Xiao, F. Macromolecules 2012, 45, 4085.
[19] He, F.; Yuan, C.; Li, K.; Diao, S.; Jin, K.; Wang, J.; Tong, J.; Ma, J.; Fang, Q. RSC Adv. 2013, 3, 23128.
[20] Ma, B.; Lauterwasser, F.; Deng, L.; Zonte, C. S.; Kim, B. J.; Fréchet, J. M. J. Chem. Mater. 2007, 19, 4827.
[21] Walker, K. A.; Markoski, L. J.; Moore, J. S. Macromolecules 1993, 26, 3713.
[22] Wang, Y.; Sun, J.; Jin, K.; Wang, J.; Yuan, C.; Tong, J.; Diao, S.; He, F.; Fang, Q. RSC Adv. 2014, 4, 39884.
[23] Chae, J. Arch. Pharm. Res. 2008, 31, 305.
[24] Kampouris, E. M. J. Chem. Soc. 1965, 2651.
[25] Yang, J.; Liu, S.; Zhu, F.; Huang, Y.; Li, B.; Zhang, L. J. Polym. Sci., Part A:Polym. Chem. 2011, 49, 381.
/
| 〈 |
|
〉 |