

Chinese Journal of Organic Chemistry >
Biocatalytic Desymmetrization of Dinitriles in Organic Synthesis
Received date: 2016-05-09
Revised date: 2016-06-11
Online published: 2016-06-20
Supported by
Project supported by the National Natural Science Foundation of China (No.21502202).
In comparison with the chemical hydration and hydrolysis of nitriles, which usually involves harsh reaction condi-tions and low selectivity, biocatalytic desymmetrizations of prochiral or meso nitriles are highly efficient, highly enantioselective and environmentally benign. Therefore, biocatalysis and biotransformation has offered an attractive and unique protocol for the enantioselective synthesis of polyfunctionalized organic compounds that are not readily obtainable by other methods. This review summarizes the biocatalytic desymmetrization of prochiral nitriles including glutaronitriles, malonitriles and meso cyclic dinitriles during the past two decades.
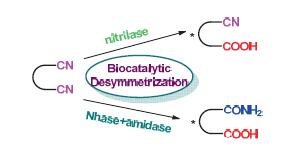
Key words: desymmetrization; biotransformations; dinitriles
Ao Yufei , Wang Qiqianga , Wang Dexiana . Biocatalytic Desymmetrization of Dinitriles in Organic Synthesis[J]. Chinese Journal of Organic Chemistry, 2016 , 36(10) : 2333 -2343 . DOI: 10.6023/cjoc201605009
[1] Faber, K. Biotransformations in Organic Chemistry, 6th ed., Springer, Berlin, 2011.
[2] Rappoport, Z.; Patai, S. The Chemistry of Functional Groups, The Chemistry of the Cyano Group, Wiley, London, 1970.
[3] (a) Evgred, D.; Harnett, S. Cyanide Compounds in Biology (Ciba Foundation Symposium 140), Wiley, Chichester, 1998. (b) Jallageas, J.-C.; Arnaud, A.; Galzy, P. Adv. Biochem. Eng. 1980, 12, 1. (c) Legras, J.-L.; Chuzel, G.; Arnaud, A.; Galzy, P. World J. Microbiol. Biotechnol. 1990, 6, 83.
[4] (a) Harper, D. B. Biochem. Soc. Trans. 1976, 4, 502. (b) Harper, D. B. Biochem. J. 1977, 165, 309. (c) Kobayashi, M.; Shimizu, S. FEBS Microbiol Lett. 1994, 120, 217.
[5] (a) Asano, Y.; Tani, Y.; Yamada, H. Agric. Biol. Chem. 1980, 44, 2251. (b) Asano, Y.; Tachibana, Y.; Tani, Y.; Yamada, H. Agric. Biol. Chem. 1982, 46, 1175.
[6] (a) Brenner, C. Curr. Opin. Struct. Biol. 2002, 12, 775. (b) Liu, Z. -Q.; Dong, L. -Z.; Cheng, F.; Xue, Y. -P.; Wang, Y. -S.; Ding, J. -N.; Zheng, Y. -G.; Shen, Y. -C. J. Agric. Food Chem. 2011, 59, 11560.
[7] (a) Mascharak, P. K. Coord. Chem. Rev. 2002, 225, 201. (b) Song, L. Y.; Wang, M. Z.; Shi, J. J.; Xue, Z. Q.; Wang, M. -X.; Qian, S. J. Biochem. Biophy. Res. Commun. 2007, 362, 319.
[8] (a) Fournand, D.; Arnaud, A. J. Appl. Microbiol. 2001, 91, 381. (b) Ohtaki, A.; Murata, K.; Sato, Y.; Noguchi, K.; Miyatake, H.; Dohmae, N.; Yamada, K.; Yohda, M.; Odaka, M. Biochim. Biophys. Acta 2010, 1804, 184.
[9] (a) Sugai, T.; Yamazaki, T.; Yokoyama, M.; Ohta, H. Biosci. Biotechnol. Biochem. 1997, 61, 1419. (b) Martínková, L.; K?en, V. Biocatal. Biotrans. 2002, 20, 73. (c) Banerjee, A.; Sharma, R. Banerjee, U. C. Appl. Microbiol. Biotechnol. 2002, 60, 33. (d) Wang, M.-X. Top. Catal. 2005, 35, 117. (e) Martínková, L.; Uhnáková, B.; Pátek, M.; Nešvera, J.; K?en, V. Rhodococcus. Environ. Int. 2009, 35, 162. (f) Wang, M.-X. Chimia 2009, 63, 331. (g) Prasad, S.; Bhalla, T. C. Biotechnol. Adv. 2010, 28, 725; (h) Velankar, H.; Clarke, K. G.; du Preez, R.; Cowan, D. A.; Burton, S. G. Trends Biotechnol. 2010, 28, 561. (i) Wang, M.-X. Top. Organomet. Chem. 2011, 36, 105. (j) Ramteke, P. W.; Maurice, N. G.; Joseph, B.; Wadher, B. J. Biotechnol. Appl. Biochem. 2013, 60, 459. (k) Wang, M.-X. Acc. Chem. Res. 2015, 48, 602.
[10] (a) Garcia-Urdiales, E.; Alfonso, I.; Gotor, V. Chem. Rev. 2005, 105, 313. (b) Palomo, J. M.; Cabrera, Z. Curr. Org. Synth. 2012, 9, 791.
[11] Kakya, H.; Sakai, N.; Yokoyama, M.; Sugai, T.; Ohta, H. Chem. Lett. 1991, 1823.
[12] (a) Crosby, J. A.; Parratt, J. S.; Turner, N. J. Tetrahedron:Asymmetry 1992, 3, 1547. (b) Beard, T.; Cohen, M. A.; Parratt, J. S.; Turner, N. J.; Crosby, J.; Moilliet, N. J. Tetrahedron:Asymmetry 1993, 4, 1085.
[13] (a) Wang, M.-X.; Liu, C.-S.; Li, J.-S. Meth-Cohn, O. Tetrahedron Lett. 2000, 41, 8549. (b) Wang, M.-X.; Liu, C.-S.; Li, J.-S. Tetrahedron:Asymmetry 2002, 12, 3367.
[14] Vink, M. K. S.; Schortinghuis, C. A.; Luten, J.; van Maarseveen, J. H.; Schoemaker, H. E.; Hiemstra, H.; Rutjes, F. P. J. T. J. Org. Chem. 2002, 67, 7869.
[15] (a) Santis, D. G.; Zhu, Z. L.; Greenberg, W. A.; Wong, K.; Chaplin, J.; Hanson, S. R.; Farwell, B.; Nicholson, L. W.; Rand, C. L.; Weiner, D. P.; Robertson, D. E.; Burk, M. J. J. Am. Chem. Soc. 2002, 124, 9024. (b) Santis, D. G.; Wong, K.; Farwell, B.; Chatman, K.; Zhu, Z. L.; Tomlinson, G.; Huang, H.; Tan, X.; Bibbs, L.; Chen, P.; Kretz, K.; Burk, M. J. J. Am. Chem. Soc. 2003, 125, 11476.
[16] Bergeron, S.; Chaplin, D. A.; Edwards, J. H.; Ellis, B. S. W.; Hill, C. L.; Karen, H.-T.; Knight, J. R.; Mahoney, T.; Osborne, A. P.; Ruecroft, G. Org. Process Res. Dev. 2006, 10, 661.
[17] Kinfe, H. H.; Chhiba, V.; Frederick, J.; Bode, M. L.; Mathiba, K.; Steenkamp, P. A.; Brady, D. J. Mol. Catal. B:Enzym. 2009, 59. 231.
[18] Xu, M. Z.; Ren, J.; Gong, J. S.; Dong, W. Y.; Wu, Q. Q.; Xu, Z. H.; Zhu, D. M. Chin. J. Biotechnol. 2013, 29, 31(in Chinese). (许美珍, 任杰, 龚劲松, 董文玥, 吴洽庆, 许正宏, 朱敦明, 生物工程学报, 2013, 29, 31.)
[19] Duan, Y. T.; Yao, P. Y.; Ren, J.; Han, C.; Li, Q.; Yuan, J.; Feng, J. H.; Wu, Q. Q.; Zhu, D. M. Sci. Chin. Chem. 2014, 57, 1164.
[20] Nojiri, M.; Uekita, K.; Ohnuki, M.; Taoka, N.; Yasohara, Y. J. Appl. Microbiol. 2013, 115, 1127.
[21] Yokoyama, M.; Sugai, T.; Ohta, H. Tetrahedron:Asymmetry 1993, 4, 1081.
[22] Yokoyama, M.; Kashiwagi, M.; Iwasaki, M.; Fuhshuku, K.; Ohta, H.; Sugai, T. Tetrahedron:Asymmetry 2004, 15, 2817.
[23] (a) Wu, Z. -L.; Li, Z. -Y. Chem. Commun. 2003, 386. (b) Wu, Z. -L.; Li, Z. -Y. J. Org. Chem. 2003, 68, 2479. (c) Wu, Z. -L.; Li, Z. -Y. Tetrahedron:Asymmetry 2003, 14, 2133.
[24] Vink, M. K. S.; Wijtmans, R.; Reisinger, C.; Berg, R. J. F.; Schortinghuis, C. A.; Schwab, H.; Schoemaker, H. E.; Rutjes, F. P. J. T. Biotechnol. J. 2006, 1, 569.
[25] Zhang, L.-B.; Wang, D.-X.; Wang, M.-X. Tetrahedron 2011, 67, 5604.
[26] Zhang, L.-B.; Wang, D.-X.; Zhao, L.; Wang, M.-X. J. Org. Chem. 2012, 77, 5584.
[27] Matoishi, K.; Sano, A.; Imai, N.; Yamazaki, T.; Yokoyama, M.; Sugai, T.; Ohta, H. Tetrahedron:Asymmetry 1998, 9, 1097.
[28] (a) Chen, P.; Gao, M.; Wang, D.-X.; Zhao, L.; Wang, M.-X. Chem. Common. 2012, 48, 3482. (b) Chen, P.; Gao, M.; Wang, D.-X.; Zhao, L.; Wang, M.-X. J. Org. Chem. 2012, 77, 4063.
[29] Ao, Y.-F.; Wang, D.-X.; Zhao, L.; Wang, M.-X. Chem. Asian J. 2015, 10, 938.
[30] (a) Kielbasinski, P.; Rachwalski, M.; Mikolajczyk, M.; Szyrej, M.; Wieczorek, M. W.; Wijtmans, R.; Rutjes, F. P. J. T. Adv. Synth. Catal. 2007, 349,1387. (b) Kielbasinski, P.; Rachwalski, M.; Kwiatkowska, M.; Mikolajczyk, M.; Wieczorek, M. W.; Szyrej, M.; Sieron, L.; Rutjes, F. P. J. T. Tetrahedron:Asymmetry 2007, 18, 2108.
[31] Fernandes, B. C. M.; Mateo, C.; Kiziak, C.; Chmura, A.; Wacker, J.; Rantwijk, F. V.; Stolz, A.; Sheldon, R. A. Adv. Synth. Catal. 2006, 348, 2597.
/
| 〈 |
|
〉 |