

Chinese Journal of Organic Chemistry >
Biocatalytic Synthesis of a Novel Corosolic Acid Derivative
Received date: 2016-05-09
Revised date: 2016-06-04
Online published: 2016-07-13
Supported by
Project supported by the National Natural Sciences Foundation of China (No.21462057),the Program of Ministry of Education "Chunhui Plan" (No.Z2014091),and the United Fund of Guizhou Province,Zunyi Medical University and Zunyi City (No.QKHLH-2014-7555).
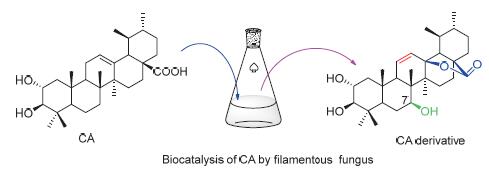
Corosolic acid, a pentacyclic triterpene acid, attracted more and more attention because of the significant anti-diabetes activity. Biocatalysis was applied to modify the structure of corosolic acid to increase the structural diversity of corosolic acid and lay the foundation for screening derivatives with novel structure or better activity. A novel corosolic acid derivative was synthesized by endophytic fungus Umbelopsis isabellina. The structure of the new compound was established by HR-ESIMS and NMR spectrum.
Fu Shaobin , Meng Qingfengb , Xin Qinga , Xiao Shijia . Biocatalytic Synthesis of a Novel Corosolic Acid Derivative[J]. Chinese Journal of Organic Chemistry, 2016 , 36(11) : 2743 -2745 . DOI: 10.6023/cjoc201605011
[1] Lin, G.-R.; Shen, G.-Y.; Wu, J.-C. Chin. Agric. Sci. Bull. 2014, 28, 133 (in Chinese). (林国荣, 沈高扬, 吴锦程, 中国农学通报, 2014, 28, 133.)
[2] He, J.-R.; Liu, J.; Bai, Z.-L.; Huang, R.-Q.; Wang, Z.-L. J. Southern Med. Univ. 2010, 11, 2533 (in Chinese). (贺建荣, 刘欠, 白志龙, 黄仁权, 王增禄, 南方医科大学学报, 2010, 11, 2533.)
[3] Sung, B.; Kang, Y. J.; Kim, D. H.; Hwang, S. Y.; Lee, Y.; Kim, M.; Yoon, J.-H.; Kim, C. M.; Chung, H. Y.; Kim, N. D. Int. J. Mol. Med. 2014, 33, 943.
[4] Kim, J.-H.; Kim, Y.-H.; Song, G.-Y.; Kim, D.-E.; Jeong, Y.-J.; Liu, K.-H.; Chung, Y.-H.; Oh, S. Food Chem. Toxicol. 2014, 67, 87.
[5] Aguirre, M. C.; Delporte, C.; Backhouse, N.; Erazo, S.; Letelier, M. E.; Cassels, B. K.; Silva, X.; Alegría, S.; Negrete, R. Bioorg. Med. Chem. 2006, 14, 5673.
[6] Xu, Y.-F.; Zhao, Y.-H.; Xu, Y.-L.; Guan, Y.; Zhang, X.; Chen, Y.; Wu, Q.; Zhu, G.-Q.; Chen, Y.-X.; Sun, F.-Y.; Wang, J.-Y.; Yu, Y.-C. Cell Signal 2016, 29, 209.
[7] Ren, X.-H.; Lu, X.-F. Prog. Pharm. Sci. 2011, 3, 129 (in Chi-nese). (任新凤, 陆雪芬, 药学进展, 2011, 3, 129.)
[8] Park, C.; Lee, J.-S. Biomed. Res. 2013, 24, 164.
[9] Giampapa, V. C.; Falls, L. WO 2006127779, 2006[Chem. Abstr. 2005, 145, 511747].
[10] Deng, J.-J.; Lu, C.-H.. Nat. Prod. Res. 2015, 30, 1
[11] Feng, X.; Li, D.-P.; Chu, Z.-Y. Nat. Prod. Res. 2014, 28, 1.
[12] Li, D.-P.; Feng, X.; Chu, Z.-Y.; Guo, F.-F.; Zhang, Z.-S. J. Asian Nat. Prod. Res. 2013, 15, 789.
[13] Fu, S.-B.; Yang, J.-S.; Cui, J.-L.; Feng, X.; Sun, D.-A. Chem. Pharm. Bull. 2011, 59, 1180.
[14] Fu, S.-B.; Yang, J.-S.; Cui, J.-L.; Sun, D.-A. Fitoterapia 2013, 86, 123.
[15] Feng, X.; Lu, Y.-H.; Liu, Z.; Li, D.-P.; Zou, Y.-X.; Fang, Y.-Q.; Chu, Z.-Y. J. Asian Nat. Prod. Res. 2016, 30, 1.
/
| 〈 |
|
〉 |