

Chinese Journal of Organic Chemistry >
Studies on the Total Synthesis of iso-L-Cystidine
Received date: 2016-05-19
Revised date: 2016-07-04
Online published: 2016-07-15
Supported by
Project supported by National Natural Science Foundation of China (No.21462019),the New Century Excellent Talents in University (No.11-1000),and the Bureau of Science&Technology of Jiangxi Province (No.20143ACB20012).
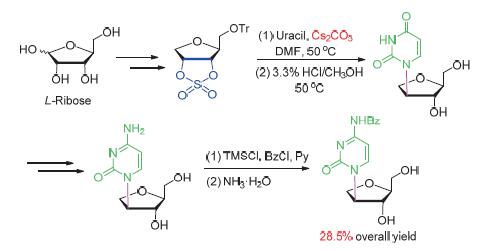
In order to systematic study the biological effects of isonucleoside incorporated oligonucleotides, an efficient synthetic process for iso-L-cystidine was investigated in present paper. Using L-ribose as starting material, iso-L-cystidine was obtained in 9 steps and 28.5% overall yield. The key step was the regioselective nucleophilic substitution of 5-O-triphenylmethane-2,3-O-cyclic sulfate with uracil. After extensive optimization, it was found that using cesium carbonate as base can improve significantly the N-1 glycosidic bond formation and reduce the side reactions. At the same time, a N-1,N-3 doublic 1-deoxyribose substituted iso-L-uridine was identified for the first time. The present reported synthetic process has the merits of mild reaction conditions, easy purified intermediates and high overall yield, which could be used as a general synthetic approach for the preparation other related isonucleoside.
Sun Zhidong , Zhu Yunlong , Huang Haiyang , Song Xianrong , Xiao Qiang . Studies on the Total Synthesis of iso-L-Cystidine[J]. Chinese Journal of Organic Chemistry, 2016 , 36(11) : 2729 -2734 . DOI: 10.6023/cjoc201605036
[1] (a) Jordheim, L. P.; Durantel, D.; Zoulim, F.; Dumontet, C. Nat. Rev. Drug Discovery 2013, 12, 447.
(b) Zhou, X.-X.; Littler, E. Curr. Top. Med. Chem. 2006, 6, 851.
(c) Huryn, D. M.; Okabe, M. Chem. Rev. 1992, 92, 1745.
(d) Wu, Y.-W.; Jiang, Y.-Y.; Fu, H.; Yang, J.; Zhao, Y.-F. Chin. J. Org. Chem. 2003, 23, 1091 (in Chinese). (吴耀文, 蒋宇扬, 付华, 杨杰, 赵玉芬, 有机化学, 2003, 23, 1091.)
(e) Fan, X.-S.; Zhang, X.-Y.; Wang, X.; Qu, G.-R. Chin. J. Org. Chem. 2008, 28, 1888 (in Chinese). (范学森, 张新迎, 王霞, 渠桂荣, 有机化学, 2008, 28, 1888.)
[2] (a) Montgomery, J. A.; Clayton, S. D.; Thomas, H. J. J. Org. Chem. 1975, 40, 1923.
(b) Montgomery, J. A.; Thomas, H. J. J. Org. Chem. 1978, 43, 541.
[3] (a) Bloch, A.; Robins, M. J.; McCarthy, J. R. J. Med. Chem. 1967, 10, 908.
(b) Nair, V.; Jahnke, T. S. Antimicrob. Agents Chemother. 1995, 39, 1017.
[4] (a) Nair, V.; Piotrowska, D. G.; Okello, M.; Vadakkan, J. Nucleos Nucleot. Nucl. 2007, 26, 687.
(b) Chun, B. K.; Vadakkan, J. J.; Nair, V. Nucleosides, Nucleotides, Nucleic Acid 2005, 24, 725.
(c) Chi, G. C.; Neamati, N.; Nair, V. Bioorg. Med. Chem. Lett. 2004, 14, 4815.
(d) Tian, X.-B.; Zhang, L.; Min, J.-M.; Zhang, L.-H.; Lu, Y.; Jiang, R.-W.; Zheng, Q.-T. Chem. Res. Chin. Univ. 2001, 17, 178.
(e) Song, Y.; Yang, R.; Ding, H.; Sun, Q.; Xiao, Q.; Ju, Y. Synthesis-Stuttgart 2011, 1213.
[5] Cai, B.; Yang, X.; Sun, L.; Fan, X.; Li, L.; Jin, H.; Wu, Y.; Guan, Z.; Zhang, L.; Zhang, L.; Yang, Z. Org. Biomol. Chem. 2014, 12, 8866.
[6] Huang, Y.; Chen, Z.; Chen, Y.; Zhang, H.; Zhang, Y.; Zhao, Y.; Yang, Z.; Zhang, L. Bioconjugate Chem. 2013, 24, 951.
[7] Okello, M, Nair, V. Chem. Synth. Nucleoside Analogues 2013, 317.
[8] Purdy, D. F.; Zintek, L. B.; Nair, V. Nucleosides Nucleotides 1994, 13, 109.
[9] Bennek, J. A.; Gray, G. R. J. Org. Chem. 1987, 52, 892.
[10] CCDC 1474741 (5) contain the supplementary crystallographic data for this paper.
[11] (a) Dijkstra, G.; Kruizinga, W. H.; Kellogg, R. M. J. Org. Chem. 1987, 52, 4230.
(b) Ostrowicki, A.; Koepp, E.; Vögtle, F. Macrocycles 1992, 37.
(c) Galli, C. Org. Prep. Proced. Int. 1992, 24, 285.
(d) Huang, H.-Y.; Sun, Z.-D.; Gong, Z.-W.; Xiao, Q.; Chen, X. Adv. Mater. Res. 2014, 997, 193.
[12] (a) Freeman, G. A.; Selleseth, D. W.; Rideout, J. L.; Harvey, R. J. Nucleosides Nucleotides Nucleic Acid 2000, 19, 155.
(b) Verheggen, I.; Aerschot, A. V.; Toppet, S.; Snoeck, R.; Janssen, G.; Balzarini, J.; Clercq, E. D.; Herdewijn, P. J. Med. Chem., 1993, 36, 2033.
[13] Blackburn, M.; Gait, M.; Loakes, D. Nucleic Acids in Chemistry and Biology, Ed.:Williams, D., Cambridge, UK, 2006.
[14] Yu, H.-W.; Zhang, L.-R.; Zhou, J.-C.; Ma, L.-T.; Zhang, L.-H. Bioorg. Med. Chem. 1996, 4, 609.
/
| 〈 |
|
〉 |