

Chinese Journal of Organic Chemistry >
A Fast and Efficient Approach for Screening and Synthesis of the Products of 2-Pyrimidinyloxy-N-arylbenzylamine Derivatives via Acid-Catalyzed Smiles Rearrangement
Received date: 2016-04-10
Revised date: 2016-06-02
Online published: 2016-08-10
Supported by
Project supported by the Agro-scientific Research in the Public Interest (No. 201403030) and the National Natural Science Foundation of China (No. 31471807).
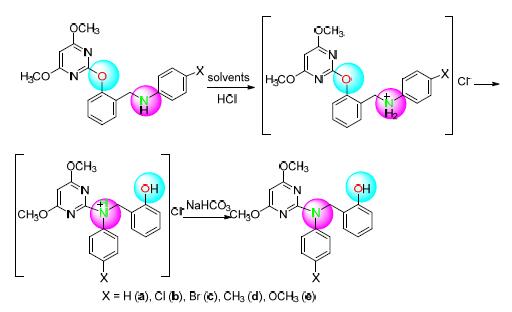
Based on high performance liquid chromatography (HPLC), a fast, efficient and simple route for Smiles rearrangement reaction has been developed in the presence of 2-pyrimidinyloxy-N-arylbenzylamine derivatives as reactants with HCl as acid catalyst in mixed CH3OH/H2O medium at room temperature. The advantage of the proposed method is that the products with excellent yields and high purity can be obtained employing reused solvent without further separating process. Considering the cheapness of catalyst and solvent, as well as high efficient and environment-friendly reaction condition, the developed method has the practicability and environmentally friendliness.
Ye Meijun , Zhang Peizhi , Wu Jun . A Fast and Efficient Approach for Screening and Synthesis of the Products of 2-Pyrimidinyloxy-N-arylbenzylamine Derivatives via Acid-Catalyzed Smiles Rearrangement[J]. Chinese Journal of Organic Chemistry, 2016 , 36(12) : 2997 -3002 . DOI: 10.6023/cjoc201604020
[1] Wu, J.; Cheng, J.; Lu, L. J. Agric. Food Chem. 2006, 54, 5954.
[2] Wu, J.; Zhang, P. Z.; Lu, L.; Yu, Q. S.; Hu, X. R.; Gu, J. M. Chin. J. Struct. Chem. 2003, 22, 613.
[3] Fan, Q.-J.; Peng, W.-L.; Shen, D.-L. J. Agrochemicals 2005, 44, 257(in Chinese).(范钱君, 彭伟立, 沈德隆, 农药, 2005, 44, 257.)
[4] Zhang, Q.; Hang, J.-H.; Yang, Z.-M.; Zhu, Q.-S.; Ye, Q.-F.; Lu, L; Xu, B.-J.; Chen, Z.-F. Acta Agric. Nucl. Sin. 2008, 22, 84(in Chinese).(张泉, 黄建中, 杨征敏, 朱其松, 叶庆富, 吕龙, 徐步进, 陈子元, 核农学报, 2008, 22, 84.)
[5] Zhang, Q. M.S. Thesis, Zhejiang University, Hangzhou, 2007(in Chinese).(张泉, 硕士论文, 浙江大学, 杭州, 2007.)
[6] Wang, H. Y.; Liao, Y. X.; Guo, Y. L.; Tang, Q. H.; Lu, L. Synlett 2005, 1239.
[7] Kitching, M. O.; Hurst, T. E.; Sniekus, V. Angew. Chem., Int. Ed. 2012, 51, 2925.
[8] Yu, J. Z.; Wang, Y. T.; Zhang, P. Z.; Wu, J. Synlett 2013, 24, 1448.
[9] Yu, J. Z.; Zhang, P. Z.; Wu, J.; Shang, Z. C. Tetrahedron Lett. 2013, 24, 3167.
[10] Liu, S. H.; Hu, Y.; Qian, P. F.; Hu, Y. W.; Ao, G. Z.; Chen, S. H.; Zhang, S. L.; Zhang, Y. N. Tetrahedron Lett. 2015, 56, 2211
[11] Nechepurenko, I. V.; Komarova, N. I.; Shernyukov, A. V.; Vasiliev, V. G.; Salakhutdinov, N. F. Tetrahedron Lett. 2014, 55, 6125
[12] Xiao, Y. X.; Zhang, Z. C.; Chen, Y. B.; Shao, X. S.; Li, Z.; Xu, X. Y. Tetrahedron 2015, 71, 1863.
[13] Takahashi, T.; Maki, Y. Chem. Pharm. Bull. 1958, 6, 369.
[14] Rodig, O. R.; Schlatze, R. K.; Collier, R. E. J. Org. Chem. 1964, 29, 2652.
[15] Sunamoto, J.; Kondo, H.; Yanase, F.; Okamoto, H. B. Chem. Soc. Jpn. 1980, 53, 1361.
[16] Lindberg, P.; Nordberg, P.; Alminger, T.; Brandstrom, A.; Wallmark, B. J. Med. Chem. 1986, 29, 1327.
[17] Terauchi, H.; Tanitame, A.; Tada, K.; Nakamura, K.; Seto, Y.; Nishikawa, Y. J. Med. Chem. 1997, 40, 313.
[18] Kuhler, T. C.; Swanson, M.; Christenson, B.; Klintenberg, A. C.; Lamm, B.; Fagerhag, J.; Gatti, V.; Elebring, T. J. Med. Chem. 2002, 45, 4282.
[19] Potashman, M. H.; Duggan, M. E. J. Med. Chem. 2009, 52, 1231.
[20] Shin, J. M.; Cho, Y. M.; Sachs, G. J. Am. Chem. Soc. 2004, 126, 7800.
[21] Kürti, L.; Czakó, B. Strategic Applications of Named Reactions in Organic Synthesis, Elsevier Academic Press, New York, 1961, pp. 417~418.
[22] Plesniak, K.; Zarecki, A.; Wicha, J. Top. Curr. Chem. 2007, 275, 163.
[23] Selvakumar, N.; Srinivas, D.; Azhagan, A. M.; Selvakumar, N. Synthesis 2002, 2421.
[24] Cho, S. D.; Park, Y. D.; Kim, J. J.; Lee, S. G.; Ma, C.; Song, S. Y.; Joo, W. H.; Falck, J. R.; Shiro, M.; Shin, D. S.; Yoon, Y. J. J. Org. Chem. 2003, 68, 7918.
[25] Mosselhi, M. A. N.; Abdallah, M. A.; Farghaly, T. A.; Shawalli, A. S. Monatsh. Chem. 2004, 135, 211.
[26] Xiang, J.; Zheng, L.; Xie, H.; Hu, X.; Dang, Q.; Bai, X. Tetrahedron 2008, 64, 9101.
[27] Yu, J.-Z. Ph.D. Dissertation, Zhejiang University, Hangzhou, 2013(in Chinese).(俞建忠, 博士论文, 浙江大学, 杭州, 2013.)
[28] Zhang, P.-Z.; Ye, M.-J.; Hu, W.-L.; Wu, J. Acta Phys.-Chim. Sin. 2016, 32, 422(in Chinese).(张培志, 叶美君, 胡伟莲, 吴军, 物理化学学报, 2016, 32, 422.)
[29] Wu, H. F.; Zhang, P. Z.; Wu, J. J. Zhejiang Univ., Sci. Ed. 2010, 11, 94.
/
| 〈 |
|
〉 |