

Chinese Journal of Organic Chemistry >
Recent Advances in Oxidative Cycloaddition Reactions of Phenols with Olefins
Received date: 2016-07-10
Revised date: 2016-08-30
Online published: 2016-09-08
Supported by
Project supported by the National Natural Science Foundation of Shaanxi Province(No. 2011JQ2001).
Phenol oxidation and the induced transformations can construct various core skeletons of bioactive molecules.Due to the extensive existence of dihydrobenzofuran core,which could be generated from the oxidative cycloaddtion reaction of phenols and olefins in bioactive neolignans and resveratrol oligomers,the oxidative cycloaddition reaction of phenols and olefins catched broad attention from organic chemists.Herein,the progress of oxidative cycloadditon reactions of phenols and olefins in recent years is reviewed according to the difference of reaction conditions,and the corresponding reaction mechanisms are discussed.
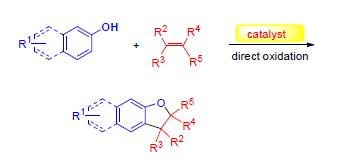
Key words: phenol; olefin; oxidative cycloaddition reaction; dihydrobenzofuran
Cui Na , Zhao Yu , Wang Yunxia . Recent Advances in Oxidative Cycloaddition Reactions of Phenols with Olefins[J]. Chinese Journal of Organic Chemistry, 2017 , 37(1) : 20 -30 . DOI: 10.6023/cjoc201607017
[1] (a) Fan, R.; Ding, Q.; Ye, Y. Synthesis 2012, 45, 1.
(b) Su, B.; Deng, M.; Wang, Q. Org. Lett. 2013, 15, 1606.
[2] (a) Libman, A.; Shalit, H.; Vainer, Y.; Narute, S.; Kozuch, S.; Pappo, D. J. Am. Chem. Soc. 2015, 137, 11453.
(b) More, N. Y.; Jeganmohan, M. Org. Lett. 2015, 17, 3042.
(c) Matsushita, M.; Kamata, K.; Yamaguchi, K.; Mizuno, N. J. Am. Chem. Soc. 2005, 127, 6632.
(d) Morimoto, K.; Sakamoto, K.; Ohnishi, Y.; Miyamoto, T.; Ito, M.; Dohi, T.; Kita, Y. Chem. Eur. J. 2013, 19, 8726.
(e) Elsler, B.; Schollmeyer, D.; Dyballa, K. M.; Franke, R.; Waldvogel, S. R. Angew. Chem., Int. Ed. 2014, 53, 5210.
(f) Lee, Y. E.; Cao, T.; Torruellas, C.; Kozlowski, M. C. J. Am. Chem. Soc. 2014, 136, 6782.
(g) Gaster, E.; Vainer, Y.; Regev, A.; Narute, S.; Sudheendran, K.; Werbeloff, A.; Shalit, H.; Pappo, D. Angew. Chem., Int. Ed. 2015, 54, 4198.
(h) Morimoto, K.; Sakamoto, K.; Ohshika, T.; Dohi, T.; Kita, Y. Angew. Chem., Int. Ed. 2016, 55, 3652.
[3] (a) Seoane, A.; Casanova, N.; Quinones, N.; Mascarenas, J. L.; Gulias, M. J. Am. Chem. Soc. 2014, 136, 7607.
(b) Zuo, Z.; Yang, X.; Liu, J.; Nan, J.; Bai, L.; Wang, Y.; Luan, X. J. Org. Chem. 2015, 80, 3349.
[4] (a) Guo, X.; Yu, R.; Li, H.; Li, Z. J. Am. Chem. Soc. 2009, 131, 17387.
(b)Parnes, R.; Kshirsagar, U. A.; Werbeloff, A.; Regev, C.; Pappo, D. Org. Lett. 2012, 14, 3324.
(c)Pappo, D.; Regev, A.; Shalit, H. Synthesis 2015, 47, 1716.
[5] (a) Denizot, N.; Pouilhes, A.; Cucca, M.; Beaud, R.; Guillot, R.; Kouklovsky, C.; Vincent, G. Org. Lett. 2014, 16, 5752.
(b) Tomakinian, T.; Guillot, R.; Kouklovsky, C.; Vincent, G. Angew. Chem., Int. Ed. 2014, 53, 11881.
(c) Vincent, G.; Beaud, R.; Tomakinian, T.; Denizot, N.; Pouilhès, A.; Kouklovsky, C. Synlett 2014, 26, 432.
[6] Xu, W.; Nachtsheim, B. J. Org. Lett. 2015, 17, 1585.
[7] Lee, H.; Yi, C. S. Eur. J. Org. Chem. 2015, 9, 1899.
[8] (a) Jacquemot, G.; Ménard, M.-A.; L'Homme, C.; Canesi, S. Chem. Sci. 2013, 4, 1287.
(b) Long, R.; Yan, X.; Wu, Z.; Li, Z.; Xiang, H.; Zhou, X. Org. Biomol. Chem. 2015, 13, 3571.
[9] (a) Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Angew. Chem., Int. Ed. 2011, 50, 586.
(b) Chong, J.; Poutaraud, A.; Hugueney, P. Plant. Sci. 2009, 177, 143.
(c) Coy Barrera, E. D.; Cuca, S.; Aacute; rez, L. E. Chem. Pharm. Bull. 2009, 57, 639.
[10] Huang, Z.; Jin, L.; Feng, Y.; Peng, P.; Yi, H.; Lei, A. Angew. Chem., Int. Ed. 2013, 52, 7151.
[11] Tomakinian, T.; Guillot, R.; Kouklovsky, C.; Vincent, G. Angew. Chem., Int. Ed. 2014, 53, 11881.
[12] (a) Liang, K.; Yang, J.; Tong, X.; Shang, W.; Pan, Z.; Xia, C. Org. Lett. 2016, 18, 1474.
(b) Liang, K.; Wu, T.; Xia, C. Org. Biomol. Chem. 2016, 14, 4690.
[13] Kshirsagar, U. A.; Regev, C.; Parnes, R.; Pappo, D. Org. Lett. 2013, 15, 3174.
[14] Gaster, E.; Vainer, Y.; Regev, A.; Narute, S.; Sudheendran, K.; Werbeloff, A.; Shalit, H.; Pappo, D. Angew. Chem., Int. Ed. 2015, 54, 4198.
[15] Meng, L.; Zhang, G.; Liu, C.; Wu, K.; Lei, A. Angew. Chem., Int. Ed. 2013, 52, 10195.
[16] Blum, T. R.; Zhu, Y.; Nordeen, S. A.; Yoon, T. P. Angew. Chem., Int. Ed. 2014, 53, 11056.
[17] Song, T.; Zhou, B.; Peng, G.W.; Zhang, Q.B.; Wu, L. Z.; Liu, Q.; Wang, Y. Chem.-Eur. J. 2014, 20, 678.
[18] Zhao, Y.; Huang, B.; Yang, C.; Li, B.; Xia, W. Synthesis 2015, 47, 2731.
[19] Wang, S.; Gates, B. D.; Swenton, J. S. J. Org. Chem. 1991, 56, 1979.
[20] Gates, B. D.; Dalidowicz, P.; Tebben, A.; Wang, S.; Swenton, J. S. J. Org. Chem. 1992, 57, 2135.
[21] (a) Bérard, D.; Jean, A.; Canesi, S. Tetrahedron Lett. 2007, 48, 8238.
(b) Bérard, D.; Giroux, M. A.; Racicot, L.; Sabot, C.; Canesi, S. Tetrahedron 2008, 64, 7537.
(c) Bérard, D.; Racicot, L.; Sabot, C.; Canesi, S. Synlett 2008, 7, 1076.
(d) Sabot, C.; Bérard, D.; Canesi, S. Org. Lett. 2008, 10, 4629.
[22] Guérard, K. C.; Sabot, C.; Beaulieu, M. A.; Giroux, M. A.; Canesi, S. Tetrahedron 2010, 66, 5893.
[23] (a) Juhász, L.; Kürti, L.; Antus, S. J. Nat. Prod. 2000, 63, 866.
(b) Wang, E.; Weinb, Y.; Kuo Y. Tetrahedron Lett. 2006, 47, 9195.
[24] Mohr, A. L.; Lombardo, V. M.; Arisco, T. M.; Morrow, G. W. Synth. Commun. 2009, 39, 3845.
[25] Dohi, T.; Nakae, T.; Toyoda, Y.; Koseki, D.; Kubo, H.; Kamitanaka, T.; Kita, Y. Heterocycles 2015, 90, 631.
[26] Chen, P. Y.; Wu, Y. H.; Hsu, M. H.; Wang, T. P.; Wang, E. C. Tetrahedron 2013, 69, 653.
[27] Alvey, L.; Prado, S.; Huteau, V.; Saint-Joanis, B.; Michel, S.; Koch, M.; Cole, S.T.; Tillequin, F.; Janin Y. L. Bioorg. Med. Chem. 2008, 16, 8264.
[28] Shang, Y.; Qian, Y.; Liu, X.; Dai, F.; Shang, X.; Jia, W.; Liu, Q.; Fang, J.; Zhou, B. J. Org.Chem. 2009, 74, 5025.
[29] (a) Sako, M.; Hosokawa, H.; Ito, T.; Iinuma, M. J. Org. Chem. 2004, 69, 2598.
(b) Takaya, Y.; Terashima, K.; Ito, J.; He, Y. H.; Tateoka, M.; Yamaguchi, N.; Niwa, M. Tetrahedron 2005, 61, 10285.
(c) Bruschi, M.; Orlandi, M.; Rindone, B.; Rummakko, P.; Zoia, L. J. Phys. Org. Chem. 2006, 19, 592.
(d) Wang, G.; Wang, H.; Capretto, D. A.; Han, Q.; Hua, R.; Yang, S. Tetrahedron 2012, 68, 5216.
(e) Althagafy, H. S.; Meza-Aviña, M. E.; Oberlies, N. H.; Croatt, M. P. J. Org. Chem. 2013, 78, 7594.
(f) Magoulas, G. E.; Papaioannou, D. Molecules 2014, 19, 19769.
[30] Youn, S. W.; Eom, J. I. J. Org. Chem. 2006, 71, 6705.
[31] (a) El-Seedi, H. R.; Yamamura, S.; Nishiyama, S. Tetrahedron 2002, 58, 7485.
(b) Kirste, A.; Schnakenburg, G.; Stecker, F.; Fischer, A.; Waldvogel, S. R. Angew. Chem., Int. Ed. 2010, 49, 971.
(c) El-Seedi, H. R.; Yamamura, S.; Nishiyama, S. Tetrahedron Lett. 2002, 43, 3301.
(d) Okada, Y.; Yshoka, T.; Koike, M.; Chiba, K. Tetrahedron Lett. 2011, 52, 4690.
(e) Chiba, K.; Fukuda, M.; Kim, S.; Kitano, Y.; Tada, M. J. Org. Chem. 1999, 64, 7654.
(f) Kim, S.; Noda, S.; Hayashi, K.; Chiba, K. Org. Lett. 2008, 10, 1827.
[32] Bhusainahalli, V. M.; Spatafora, C.; Chalal, M.; Vervandier-Fasseur, D.; Meunier, P.; Latruffe, N.; Tringali, C. Eur. J. Org. Chem. 2012, 5217.
[33] (a) Nascimento, I. R.; Lopes, L. M. X.; Davin, L. B.; Lewis, N. G. Tetrahedron 2000, 56, 9181.
(b) Pereira, A. C.; Magalhaes, L. G.; Goncalves, U. O.; Luz, P. P.; Moraes, A. C. G.; Rodrigues, V.; Da Matta Guedes, P. M.; Da Silva Filho, A. A.; Cunha, W. R.; Bastos, J. K.; Nanayakkara, N. P. D.; E Silva, M. L. Phytochemistry 2011, 72, 1424.
(c) Syrjänen, K.; Brunow, G. Tetrahedron 2001, 57, 365.
(d) Toshiyuki,W.; Makoto, Nitta.; Kana, K.; Taketo, C.; Ye, Y.; Kuniro, T.; Toshiyuki, K.; Haruo, N.; Masaji, I.; Minako, K.; Yoshiaki, Y.; Yoshihide, S. Bioorg. Med. Chem. Lett. 2009, 19, 5905.
(e) Li, C.; Lu, J.; Xu, X.; Hu, R.; Pan, Y. Green Chem. 2012, 14, 328.
/
| 〈 |
|
〉 |