

Chinese Journal of Organic Chemistry >
Synthesis, Antibacterial Activity and Structure-Activity Relationship of 1H-2,5-Dihydro-1,5-benzodiazepine with Ester Group and the Aromatic Heterocyclic Ring
Received date: 2016-04-29
Revised date: 2016-05-23
Online published: 2016-10-08
Supported by
Project supported by the National Natural Science Foundation of China (No. 21276064) and the Natural Science Foundation of Hebei Province (No. B2016205165).
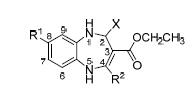
Using (ethyl 4-methyl-2-(thiazol-2-yl)-1H-2,5-dihydro-1,5-benzodiazepine-3-carboxylate) (A) as a model compound, thirty 1H-2,5-dihydro-1,5-benzodiazepine derivatives 2~6 were synthesized by nucleophilic addition reaction, elimination reaction and cyclization reaction. The structures of these new compounds were characterized by 1H NMR, 13C NMR, IR, MS and elemental analysis. The antibacterial activities of those compounds were screened using the disk diffusion method against C. neoformans, C. neoformans clinical isolates, C. albicans, E. coli and S. aureus. The bioactive assay results revealed that most of the 1H-2,5-dihydro-1,5-benzodiazepine derivatives exhibited considerable potency against all of the tested strains. In particular, some compounds exhibited excellent antimicrobial activities against the test microorganism MS except E. coli, and exhibited better antimicrobial activity against fungi than against bacteria. Furthermore, ethyl 4-ethyl-8-methyl-2-(thiazol-2-yl)-2,5-dihydro-1,5-benzodiazepine-3-carboxylate (4b) and ethyl 4-ethyl-8-fluoro-2-(thiazol-2-yl)-2,5-dihydro-1,5-benzo-diazepine-3-carboxylate (4c), which had good antibacterial activities, were subjected to further pharmacological evaluation, including minimum inhibitory concentration (MIC), minimum fungicidal concentration (MFC) and minimum bactericidal concentration (MBC) against C. neoformans, C. albicans, and S. aureus. The results showed that the MIC and MFC against C. albicans values for above compounds were much lower than those of fluconazole. A further study of the structure-activity rela-tionship revealed that the thiazole ring at C-2, the methyl, ethyl at C-4 and the methyl at C-8 are essential for antibacterial activity.
Zhang Xiujun , Wang Lanzhi , Yan Jingyi , Gao Chen . Synthesis, Antibacterial Activity and Structure-Activity Relationship of 1H-2,5-Dihydro-1,5-benzodiazepine with Ester Group and the Aromatic Heterocyclic Ring[J]. Chinese Journal of Organic Chemistry, 2017 , 37(2) : 462 -473 . DOI: 10.6023/cjoc201604059
[1] Francisco, L. M.; Cecilio, Á.; Pilar, G. G. J. Anxiety Disord. 2011, 25, 554.
[2] Konda, S. G.; Shaikh, B. M.; Chavan, S. A.; Dawane, B. S. Chin. Chem. Lett. 2011, 22, 65.
[3] Ganzella, M.; Faraco, R. B.; Almeida, R. F.; Fernandes, V. F.; Souza, D. O. Pharmacol. Biochem. Behav. 2011, 100, 271.
[4] Zhou, X.; Zhang, M. Y.; Gao, S. T.; M, J. J.; W, C.; Liu, C. Chin. Chem. Lett. 2009, 20, 905.
[5] Holbrook, A. M.; Crowther, R.; Lotter, A.; Cheng, C.; King, D. Can. Med. Assoc. J. 2000, 162, 225.
[6] Guzen, K. P.; Cella, R.; Stefani, H. A. Tetrahedron Lett. 2006, 47, 8133.
[7] Corral, C.; Lissavetzky, J.; Valdeolmillos, A. M. J. Heterocycl. Chem. 1985, 22, 1349.
[8] Babu, M.; Pitchumani, K.; Ramesh, P. Med. Chem. Res. 2014, 23, 2070.
[9] Ilango, S. S.; Remya, P. U.; Ponnuswamy, S. Indian J. Chem. 2013, 52B, 136.
[10] Wang, L. Z.; Li, X. Q.; An, Y. S. Org. Biomol. Chem. 2015, 13, 5497.
[11] Xiao, L. W.; Zhang, M.; Sun, W. H. Chem. Res. Chin. Univ. 2011, 27, 228.
[12] Binay, B.; Sessionsc, R. B.; Karagüler, N. G. Enzyme Microb. Technol. 2013, 52, 393.
[13] Kantevari, S.; Vuppalapati, S. V. N.; Biradar, D. O.; Nagarapu, L. J. Mol. Catal. A: Chem. 2007, 266, 109.
[14] Li, X. Q.; Wang, L. Z. Chin. Chem. Lett. 2014, 25, 327.
[15] Pasquale, G. A.; Ruiz, D. M.; Jios, J. L.; Autino, J. C.; Romanelli, G. P. Tetrahedron Lett. 2013, 54, 6574.
[16] Arshad, A.; Osman, H.; Bagley, M. C.; Lam, C. K.; Mohamad, S.; Zahariluddin, A. S. M. Eur. J. Med. Chem. 2011, 46, 3788.
[17] Sidoryk, K.; Jaromin, A.; Edward, J. A.; Switalska, M.; Stefanska, J.; Cmoch, P.; Zagrodzka, J.; Szczepek, W.; Czoch, W. P.; Wietrzyk, J.; Kozubek, A.; Zarnowski, R.; Andes, D. R.; Kaczmarek, L. Eur. J. Med. Chem. 2014, 78, 304.
/
| 〈 |
|
〉 |