

Chinese Journal of Organic Chemistry >
Synthesis and Cell Division Cycle 25B Phosphatase and Protein Ty-rosine Phosphatase 1B Inhibitory Activity Evaluation of Novel 3,6-Disubstituted Triazolothiadiazole Derivatives
Received date: 2016-07-20
Revised date: 2016-08-30
Online published: 2016-10-08
Supported by
Project supported by the Natural Science Foundation of Liaoning Province (No. 20102126).
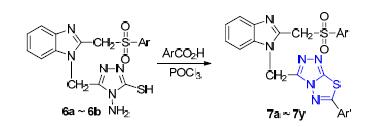
A series of new 3,6-disubstituted triazolothiadiazole derivatives 7a~7y containing benzimidazole and arylsulfonyl moities have been synthesized by o-phenylenediamine and chloroacetic acid as starting materials via multistep reactions.The structures of the intermediates 3,4,6 and the target compounds 7 were characterized by 1H NMR,IR spectra and elemental analysis.All synthesized target compounds were screened for their inhibitory activity against cell division cycle 25B phosphatase (Cdc25B) and protein tyrosine phosphatase 1B (PTP1B).The results show that some of them display significant inhibitory activities against Cdc25B and PTP1B.Among them,compound 7d exhibits the highest inhibitory activity against Cdc25B [IC50=(7.72±0.73)μg/mL]and 7u exhibits the highest inhibitory activity against PTP1B[IC50=(3.31±0.57)μg/mL].It is noteworthy that compounds 7b,7d,7l,7t and 7u show higher inhibitory activities against Cdc25B and PTP1B.They can be considered as potential Cdc25B and PTP1B inhibitors,and have great application prospects in the treatment of cancers and diabetes.
Li Yingjun , Li Jiyang , Peng Lin , Gao Lixin , Jin Kun , Sheng Li , Zhang Nan , Wang Siyuan , Li Jia . Synthesis and Cell Division Cycle 25B Phosphatase and Protein Ty-rosine Phosphatase 1B Inhibitory Activity Evaluation of Novel 3,6-Disubstituted Triazolothiadiazole Derivatives[J]. Chinese Journal of Organic Chemistry, 2017 , 37(2) : 485 -495 . DOI: 10.6023/cjoc201607030
[1] Ramaprasad, G. C.; Kalluraya, B.; Sunil, K. B. Med. Chem. Res. 2014, 23, 3644.
[2] Kamel, M. M.; Abdo, N. Y. M. Eur. J. Med. Chem. 2014, 86, 75.
[3] Ramaprasada, G. C.; Kalluraya, B.; Kumar, B. S.; Mallya, S. Pharma Chem. 2012, 49, 1026.
[4] Sunil, D.; Isloor, A. M.; Shetty, P.; Satyamoorthy, K.; Prasad, A. S. B. Med. Chem. Res. 2011, 20, 1074.
[5] Badr, S. M. I.; Barwa, R. M. Bioorg. Med. Chem. 2011, 19, 4506.
[6] Kotaiah, Y.; Nagaraju, K.; Harikrishna, N.; Rao, C. V.; Yamini, L.; Vijjulatha, M. Eur. J. Med. Chem. 2014, 75, 195.
[7] Hunashal, R. D.; Satyanarayana, D. Int. J. Pharma Bio. Sci. 2012, 3, 183.
[8] Prasad, D. J.; Ashok, M.; Karegoudar, P.; Poojary, B.; Holla, B. S. Kumari, N. S. Eur. J. Med. Chem. 2009, 44, 551.
[9] Akhter, M. W.; Hassan, M. Z.; Amir, M. Arabian J. Chem. 2014, 7, 955.
[10] Malladi, S.; Isloor, A. M.; Shetty, P.; Fun, H. K.; Telkar, S.; Mahmood, R.; Isloor, N. Med. Chem. Res. 2012, 21, 3272.
[11] Husain, A.; Naseer, M. A. Med. Chem. Res. 2011, 20, 47.
[12] Sujith, K. V.; Kalluraya, B.; Adhikari, A.; Vijayanarayana, K. Med. Chem. Res. 2012, 21, 543.
[13] Khan, I.; Ibrar, A.; Zaib, S.; Ahmad, S.; Furtmann, N.; Hameed, S.; Simpson, J.; Bajorath, J.; Iqbal, J. Bioorg. Med. Chem. 2014, 22, 6163.
[14] Khan, I.; Ibrar, A.; Abbas, N. Eur. J. Med. Chem. 2013, 63, 854.
[15] Yadav, G.; Ganguly, S. Eur. J. Med. Chem. 2015, 97, 419.
[16] Ranjith, P. K.; Rajeesh, P.; Haridas, K. R.; Susanta, N. K.; Row, T. N. G.; Rishikesan, R.; Kumari, N. S. Bioorg. Med. Chem. Lett. 2013, 23, 5228.
[17] Gupta, S. K.; Pancholi, S. S. Pharma Chem. 2011, 3, 274.
[18] Al-Mohammed, N. N.; Alias, Y.; Abdullah, Z.; Shakir, R. M.; Taha, E. M.; Hamid, A. A. Molecules 2013, 18, 11978.
[19] Gijsen, H. J. M.; Cleyn, M. A. J. D.; Surkyn, M.; Van Lommen, G. R. E.; Verbist, B. M. P. Bioorg. Med. Chem. Lett. 2012, 22,547.
[20] Travins, J. M.; Bernotas, R. C.; Kaufman, D. H.; Quinet, E.; Nambi, P.; Feingold, I.; Huselton, C.; Wilhelmsson, A.; Goos-Nilsson, A.; Wrobel, J. Bioorg. Med. Chem. Lett. 2010, 20, 526.
[21] Zhao, F.; Wang, J.; Ding, X.; Shu, S.-J.; Liu, H. Chin. J. Org. Chem. 2016, 36, 490 (in Chinese).(赵飞, 王江, 丁晓, 舒双杰, 柳红, 有机化学, 2016, 36, 490.)
[22] Mei, W.-W.; Guo, Y.-W.; Li, J.; Cai, M.-Y.; Ma, W.-Q.; Gong, J.-X.; Wang, X.-D. Chin. J. Org. Chem. 2016, 36, 533 (in Chinese).(梅雯雯, 郭跃伟, 李 佳, 蔡妹艺, 马文泉, 龚景旭, 王学东, 有机化学, 2016, 36, 533.)
[23] Li, Y.-J.; Shi, X.-L.; Gao, L.-X.; Jin, K.; Sheng, L.; Wu, J.-H.; Peng L-N; Li, J. Chin. J. Org. Chem. 2015, 35, 191 (in Chinese).(李英俊, 史相玲, 高立信, 靳焜, 盛丽, 吴疆红, 彭丽娜, 李佳, 有机化学, 2015, 35, 191.)
[24] Li, Y.-J.; Yu, Y.; Jin, K.; Gao, L.-X.; Luo, T.-C.; Sheng, L.; Sao. X.; Li, J. Chin. J. Org. Chem. 2015, 35, 129 (in Chinese).(李英俊, 于洋, 靳焜, 高立信, 罗潼川, 盛丽, 邵昕, 李佳, 有机化学, 2015, 35, 129.)
[25] Li, Y. J.; Yu, Y.; Jin, K.; Gao, L. X.; Luo, T. C.; Sheng, L.; Shao, X.; Li, J. Bioorg. Med. Chem. Lett. 2014, 24, 4125.
[26] Srinicas, K.; Dubey, P. K. Chem. Sci. Trans. 2014, 3, 375.
[27] Srinivas, K.; Dubey, P. K. Synth. Commun. 2011, 41, 1584.
[28] Dubey, P. K.; Reddy, P. V. V.; Srinivas, K. J. Heterocycl. Chem. 2010, 47, 1317.
[29] Liu, S. L.; Dong, X. Y.; Wei, F.; Wang, X.; Lv, X.; Zhong, J.; Wu, L.; Quek, S. Y.; Chen, H. Ultrason. Sonochem. 2015, 23, 100.
[30] Srinivas, K.; Dubey, P. K. Adv. Appl. Sci. Res. 2014, 5, 336.
[31] Darehkordi, A.; Ghazi, S. Arabian J. Chem. 2015, DOI: 10.1016/j.arabjc.2015.01.010.
[32] Li, Y.-J.; Liu, L.-J.; Jin, K.; Sun, S.-Q.; Xu, Y.-T. Acta Chim. Sinica 2010, 68, 1577 (in Chinese).(李英俊, 刘丽军, 靳焜, 孙淑琴, 许永廷, 化学学报, 2010, 68, 1577.)
[33] Raban, M.; Chang, H.; Craine, L.; Hortelano, E. J. Org. Chem. 1985, 50, 2205.
[34] Huang, W. G.; Jiang, Y. Y.; Li, Q.; Li, J.; Li, J. Y.; Lu, W.; Cai, J. C. Tetrahedron 2005, 61, 1863.
[35] Sun, L. P.; Shen, Q.; Piao, H. H.; Ma, W. P.; Gao, L. X.; Zhang, W.; Nan, F. J.; Li, J.; Piao, H. R. Eur. J. Med. Chem. 2011, 46, 3630.
/
| 〈 |
|
〉 |