

Chinese Journal of Organic Chemistry >
Recent Advances of Transition Metal-Catalyzed P-C Coupling Reactions
Received date: 2016-08-29
Revised date: 2016-09-26
Online published: 2016-10-11
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21302127, 21502116).
Organophosphorus compounds which contain P-C bonds have been widely used in photoelectric materials, retardant materials and medicinal chemistry. It is an important method for the synthesis of functional organophosphorus compounds from P(O)-H reagents using transition metal-catalyzed cross coupling reaction to form P-C bond. The recent development in this area is summarized on the basis of different types of carbon atom.
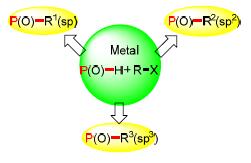
Key words: transition metal-catalyzed; P-C bond; cross-coupling
Shao Changwei , Xu Weigang , Li Liang , Zhang Xinghua . Recent Advances of Transition Metal-Catalyzed P-C Coupling Reactions[J]. Chinese Journal of Organic Chemistry, 2017 , 37(2) : 335 -348 . DOI: 10.6023/cjoc201608030
[1] Baumgartner, T.; Réau, R. Chem. Rev. 2006, 106, 4681.
[2] White, A. K.; Metcalf, W. W. Annu. Rev. Microbiol. 2007, 61, 379.
[3] Metcalf, W. W.; van der Donk, W. A. Annu. Rev. Biochem. 2009, 78, 65.
[4] De Clercq, E. Med. Res. Rev. 2010, 30, 667.
[5] De Clercq, E. Med. Res. Rev. 2011, 31, 118.
[6] Dang, Q.; Kasibhatla, S. R.; Xiao, W.; Liu, Y.; DaRe, J.; Taplin, F.; Reddy, K. R.; Scarlato, G. R.; Gibson, T.; van Poelje, P. D.; Potter, S. C.; Erion, M. D. J. Med. Chem. 2010, 53, 441.
[7] Maryanoff, B. E. J. Med. Chem. 2004, 47, 769.
[8] Kumar, T. S.; Zhou, S.-Y.; Joshi, B. V.; Balasubramanian, R.; Yang, T.; Liang, B.-T.; Jacobson, K. A. J. Med. Chem. 2010, 53, 2562.
[9] Kang, S.-U.; Shi, Z.-D.; Worthy, K. M.; Bindu, L. K.; Dhar-mawardana, P. G.; Choyke, S. J.; Bottaro, D. P.; Fisher, R. J.; Burke, T. R., Jr. J. Med. Chem. 2005, 48, 3945.
[10] Haemers, T.; Wiesner, J.; Van Poecke, S.; Goeman, J.; Henschker, D.; Beck, E.; Jomaa, H.; Van Calenbergh, S. Bioorg. Med. Chem. Lett. 2006, 16, 1888.
[11] Seto, H.; Kuzuyama, T. Nat. Prod. Rep. 1999, 16, 589.
[12] Schwan, A. L. Chem. Soc. Rev. 2004, 33, 218.
[13] Glueck, D. S. Top. Organomet. Chem. 2010, 31, 65.
[14] Tappe, F. M. J.; Trepohl, V. T.; Oestreich, M. Synthesis 2010, 3037.
[15] (a) Rajeshwaran, G. G.; Nandakumar, M.; Sureshbabu, R.; Mohanakrishnan, A. K. Org. Lett. 2011, 13, 1270.
(b) Barney, R. J.; Richardson, R. M.; Wiemer, D. F. J. Org. Chem. 2011, 76, 2875.
[16] Cohen, R. J.; Fox, D. L.; Eubank, J. F.; Salvatore, R. N. Tetrahedron Lett. 2003, 44, 8617.
[17] Lavén, G.; Stawinski, J. Synlett 2009, 225.
[18] Baslé, O.; Li, C.-J. Chem. Commun. 2009, 4124
[19] Han, W.; Ofial, A. R. Chem. Commun. 2009, 6023.
[20] Cheng, M.-X.; Ma, R.-S.; Yang, Q.; Yang, S.-D. Org. Lett. 2016, 18, 3262.
[21] Zhang, P.-B.; Zhang, L.-L.; Gao, Y.-Z.; Xu, J.; Fang, H.; Tang, G.; Zhao, Y.-F. Chem. Commun, 2015, 51, 7839.
[22] (a) Rueping, M.; Zhu, S.-Q; Koenigs, R. M. Chem. Commun. 2011, 47, 8679.
(b) Hari, D. P.; König, B. Org. Lett. 2011, 13, 3852.
(c) Xue, Q.-C.; Xie, J.; Jin, H.-M.; Cheng, Y.-X.; Zhu, C.-J. Org. Biomol. Chem. 2013, 11, 1606.
(d) Wang, X.-Z.; Meng, Q.-Y.; Zhong, J.-J.; Gao, X.-W.; Lei, T.; Zhao, L.-M.; Li, Z.-J.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Chem. Commun. 2015, 51, 11256.
(e) Rueping, M.; Zoller, J.; Fabry, D. C.; Poscharny, K.; Koenigs, R. M.; Weirich, T. E.; Mayer, J. Chem. Eur. J. 2012, 18, 3478.
[23] (a) Xu, J.; Li, X.-Q.; Gao, Y.-Z.; Zhang, L.-L; Chen, W.-Z.; Fang, H.; Tang, G.; Zhao, Y.-F. Chem. Commun. 2015, 51, 11240.
(b) Gao, Y.-Z.; Li, X.-Q.; Xu, J.; Wu, Y.-L.; Chen, W.-Z.; Tang, G.; Zhao, Y.-F. Chem. Commun. 2015, 51, 1605.
[24] Wu, J.; Gao, Y.-Z.; Zhao, X.; Zhang, L.-L; Chen, W.-Z.; Tang, G.; Zhao, Y.-F. RSC Adv. 2016, 6, 303.
[25] (a) Tappe, F. M. J.; Trepohl, V. T.; Oestreich, M. Synthesis 2010, 3037.
(b) Han, L.-B.; Tanaka, M. Chem. Commun. 1999, 395.
(c) Coundray, L.; Montchamp, J. Eur. J. Org. Chem. 2008, 3601.
(d) Prim, D.; Campagne, J.; Joseph, D.; Andrioletti, B. Tetrahedron 2002, 58, 2041.
(e) Schwan, A. L. Chem. Soc. Rev. 2004, 33, 218.
(f) Glueck, D. S. Synlett 2007, 2627.
[26] Seto, H.; Kuzuyama, T. Nat. Prod. Rep. 1999, 16, 589.
[27] (a) Glueck, D. S. Top. Organomet. Chem. 2010, 31, 65.
(b) Niu, M.; Fu, H.; Jiang, Y.; Zhao, Y. Chem. Commun. 2007, 272.
(c) Han, L.-B.; Tanaka, M. J. Am. Chem. Soc. 1996, 118, 1571.
(d) Han, L.-B.; Ono, Y.; Shimada, S. J. Am. Chem. Soc. 2008, 130, 2752.
[28] Hirao T.; Masunaga T.; Ohshiro Y.; Agawa. T. Tetrahedron Lett. 1980, 21, 3595.
[29] Hirao, T.; Masunaga, T.; Ohshiro, Y.; Agawa, T. Synthesis 1981, 56.
[30] Hirao T.; Masunaga T.; Yamada N, Ohshiro, Y.; Agawa, T. Bull. Chem. Soc. Jpn. 1982, 55, 909.
[31] Kalek, M.; Stawinski, J. Organometallics 2007, 26, 5840.
[32] Xu, Y.-Y.; Zhang, J. Synthesis 1984, 778.
[33] Bulot, J. J.; Aboujaoude, E. E.; Collignon N.; Savignac, P. Phosphorus Sulfur Relat. Elem. 1984, 21, 197.
[34] Xu, Y.-Y.; Li, Z.; Xia, J.-Z.; Guo, H.; Huang, Y.-Z. Synthesis 1984, 781.
[35] Xu, Y.-Y.; Zhang, J. J. Chem. Soc., Chem. Commun. 1986, 1606.
[36] Lu, X.-Y.; Zhu, J.-Y. Synthesis 1987, 726
[37] Zhang, J.; Xu, Y.-Y.; Huang, G.-H.; Guo, H. Tetrahedron Lett. 1988, 29, 1955.
[38] Petrakis, K. S.; Nagabhushan, T. L. J. Am. Chem. Soc. 1987, 109, 2831.
[39] Holt, D.A.; Erb, J. M. Tetrahedron Lett. 1989, 30, 5393.
[40] Xu, Y.-Y.; Wei, H.-X.; Zhang, J.; Huang, G.-H. Tetrahedron Lett. 1989, 30, 949.
[41] Bennett, S. N. L.; Hall, R. G. J. Chem. Soc., Perkin Trans. 1 1995, 1145.
[42] Trost, B. M.; Radinov, R. J. Am. Chem. Soc. 1997, 119, 5962.
[43] Machnitzki, P.; Nickel, T.; Stelzer, O.; Landgrafe, C. J. Inorg. Chem. 1998, 1029.
[44] Kazankova, M. A.; Trostyanskaya, I. G.; Lutsenko, S. V.; Beletskaya, I. P. Tetrahedron Lett 1999, 40, 569.
[45] Hall, R. G.; Riebli, P. Synlett 1999, 1633.
[46] Zhong, P.; Xiong, Z.-X.; Huang, X. Synth. Commun. 2000, 30, 273.
[47] Schuman, M.; Lopez, X.; Karplus, M.; Gouvemeur, V. Tetrahedron. 2001, 57, 10299.
[48] Kant, M.; Bischoff, S.; Siefken, R.; Gründemann, E.; Köckritz, A. J. Org. Chem. 2001, 477.
[49] Kobayashi, Y.; William, A.; Tokoro, Y. J. Org. Chem. 2001, 66, 7903.
[50] Kim, Y.-C.; Brown, S. G.; Harden, T. K.; Boyer, J. L.; Dubyak, G.; King, B. F.; Burnstock, G.; Jacobson, K. A. J. Med. Chem. 2001, 44, 340.
[51] Cristau, H.-J.; Hervé, A.; Loiseau, F.; Virieux, D. Synthesis 2003, 2216.
[52] Xie, J.-H.; Wang, L.-X.; Fu, Y.; Zhu, S.-F.; Fan, B.-M.; Duan, H.-F.; Zhou, Q.-L. J. Am. Chem. Soc. 2003, 125, 4404.
[53] Muthukumaran, K.; Loewe, R. S.; Ambroise, A.; Tamaru, S. I.; Li, Q.; Mathur, G.; Bocian, D. F.; Misra, V. J. Org. Chem. 2004, 69, 1444.
[54] Kobayashi, Y.; William, A. D. Adv. Synth. Catal. 2004, 346, 1749.
[55] Ziessel, R. F.; Charbonnière, L. J.; Mameri, S.; Camerel, F. J. Org. Chem. 2005, 70, 9835.
[56] Chauhan, S. S.; Varshney, A.; Verma, B.; Pennington, M. W. Tetrahedron 2007, 48, 4051.
[57] Bennett, J. A.; Hope, E. G.; Singh, K.; Stuart, A. M. J. Fluorine Chem. 2009, 130, 615.
[58] Kotek, J.; Hermann, P. L. V.; Muller, R. N.; Peters, J. A. J. Mater. Chem. 2009, 19, 1494.
[59] Ghalib, M.; Niaz, B.; Jones, P. G.; Heinicke, J. W. Tetrahedron Lett. 2012, 53, 5012.
[60] Rankic, D. A.; Parvez, M.; Keay, B. A. Tetrahedron: Asymmetry 2012, 23, 754.
[61] Deal, Er. L.; Petit, C.; Montchamp, J. L. Org. Lett. 2011, 13, 3270.
[62] Andaloussi, M.; Lindh, J.; Sävmarker, J.; Sjöberj, P. J. R.; Larhed, M. Chem. Eur. J. 2009, 15, 13069.
[63] Hou, C.-D.; Ren, Y.-L.; Lang, R.; Hu, X.-X.; Xia, C.-G.; Li, F.-W. Chem. Commun. 2012, 48, 5181.
[64] Mi, X.; Huang, M.-M.; Zhang, J.-Y.; Wang, C.-Y.; Wu, Y.-J. Org. Lett. 2013, 15, 6266.
[65] Feng, C.-G.; Ye, M.-C.; Xiao, K.-J.; Li, S.-H.; Yu, J.-Q. J. Am. Chem. Soc. 2013, 135, 9322.
[66] Xu, W.-T.; Hu, G.-B.; Xu, P.-X.; Gao, Y.-X.; Yin, Y.-W.; Zhao, Y.-Z. Adv. Synth. Catal. 2014, 356, 2948.
[67] Van Allen, D.; Venkataraman, D. J. Org. Chem. 2003, 68, 4590.
[68] Huang, C.; Tang, X.; Fu, H.; Jiang, Y.-Y.; Zhao, Y.-F. J. Org. Chem. 2006, 71, 5020.
[69] Zhuang, R.-Q.; Xu, J.; Cai, Z.-S.; Tang, G.; Fang, M.-J.; Zhao, Y.-F. Org. Lett. 2011, 13, 2110.
[70] Evano, G.; Tadiparthi, K.; Couty, F. Chem. Commun. 2011, 47, 179.
[71] Hu, J.; Zhao, N.; Yang, B.; Wang, G.; Guo, L.-N.; Liang, Y.-M.; Yang, S.-D. Chem. Eur. J. 2011, 17, 5516.
[72] Hu, G.-B.; Gao, Y.-X.; Zhao, Y.-F. Org. Lett. 2014, 16, 4464.
[73] Li, L.-J.; Hao, G.-L.; Zhu, A.-L.; Fan, X.-C.; Zhang, G.-S.; Zhang, L.-H. Chem. Eur. J. 2013, 19, 14403.
[74] Shen, R.-W.; Luo, B.; Yang, J.-L.; Zhang, L.-X.; Han, L.-B. Chem. Commun. 2016, 52, 6451.
[75] Han, L.-B.; Zhang, C.; Yazawa, H.; Shimada, S. J. Am. Chem. Soc. 2004, 126, 5080.
[76] Zhang, X.-H.; Liu, H.-Z.; Hu, X.-M.; Tang, G.; Zhu, J.; Zhao, Y.-F. Org. Lett. 2011, 13, 3478.
[77] Liu, L.; Wang, Y.-L.; Zeng, Z.-P.; Xu, P.-X.; Gao, Y.-X.; Yin, Y.-W.; Zhao, Y.-F. Adv. Synth. Catal. 2013, 355, 659.
[78] Hu, G.-B.; Chen, W.-Z.; Fu, T.-T.; Peng, Z.-M.; Qiao, H.-W.; Gao, Y.-X.; Zhao, Y.-F. Org. Lett. 2013, 15, 5362.
[79] Wu, Y.-L.; Liu, L.; Yan, K.-L.; Xu, P.-X.; Gao, Y.-X.; Zhao, Y.-F. J. Org. Chem. 2014, 79, 8118.
[80] Yang, G.-Q.; Shen, C.-R.; Zhang, L.; Zhang, W.-B. Tetrahedron Lett. 2011, 52, 5032.
[81] Zhao, Y.-L.; Wu, G.-J.; Han, F.-S. Chem. Commun. 2012, 48, 5868.
[82] Yang, J.; Chen, T.-Q.; Han, L.-B. J. Am. Chem. Soc. 2015, 137, 1782.
[83] Xiang, C.-B.; Bian, Y.-J.; Mao, X.-R.; Huang, Z.-Z. J. Org. Chem. 2012, 77, 7706.
[84] Gui, Q.-W.; Hu, L.; Chen, X.; Liu, J.-D.; Tan, Z. Chem. Commun. 2015, 51, 13922.
[85] Huang, X.-F.; Wu, Q.-L.; He, J.-S.; Huang, Z.-Z. Org. Biomol. Chem. 2015, 13, 4466.
[86] Gao, Y.-Z.; Lu, G.-Z.; Zhang, P.-B.; Zhang, L.-L.; Tang, G.; Zhao, Y.-F. Org. Lett. 2016, 18, 1242.
[87] Xu, W.; Zou, J.-P.; Zhang, W. Tetrahedron Lett. 2010, 51, 2639.
[88] Pan, X.-Q.; Zou, J.-P.; Zhang, G.-L.; Zhang, W. Chem. Commun. 2010, 46, 1721.
[89] Lera, M.; Hayes, C. J. Org. Lett. 2000, 2, 3873.
[90] Bernoud, E.; Alayrac, A.; Delacroix, O.; Gaumont, A. C. Chem. Commun. 2011, 47, 3239.
[91] Gao, Y.-X.; Wang, G.; Chen, L.; Xu, P.-X; Zhao, Y.-F.; Zhou, Y.-B.; Han, L.-B. J. Am. Chem. Soc. 2009, 131, 7956.
[92] Liu, P.; Yang, J.; Li, P.-H.; Wang, L. Appl. Organometal. Chem. 2011, 25, 830.
[93] Qu, Z.-B.; Chen, X.-L.; Yuan, J.-W.; Qu, L.-B.; Wang, F.-J.; Ding, X.-L.; Zhao, Y.-F. Can. J. Chem. 2012, 90, 747.
[94] Liu, L.; Wu, Y.-L.; Wang, Z.-S.; Zhu, J.; Zhao, Y.-F. J. Org. Chem. 2014, 79, 6816.
[95] Yang, J.; Chen, T.-Q.; Zhou, Y.-B.; Yin, S.-F.; Han, L.-B. Chem. Commun. 2015, 51, 3549.
[96] Wang, T.; Chen, S.-T.; Shao, A.-L.; Gao, M.; Huang, Y.-F.; Lei, A.-W. Org. Lett. 2015, 17, 118.
[97] Li, X.; Sun, S.-Y.; Yang, F.; Kang, J.-X.; Wu, Y.-S.; Wu, Y.-J. Org. Biomol. Chem. 2015, 13, 2432.
[98] Li, X.; Yang, F.; Wu, Y.-J.; Wu, Y.-S. Org. Lett. 2014, 16, 992.
/
| 〈 |
|
〉 |