

Chinese Journal of Organic Chemistry >
Design, Synthesis and Bioactivities of Novel Strobilurin Derivatives Containing 1,3,4-Oxadiazole Moity
Received date: 2016-07-19
Revised date: 2016-09-12
Online published: 2016-10-18
Supported by
Project by the “111” Project of Ministry of Education of China (No. B06005), the State Key Laboratory of Elemento-organic Chemistry and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin).
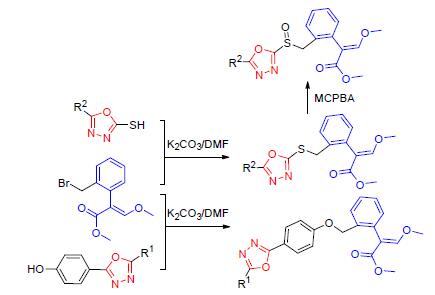
A series of novel strobilurin derivatives containing 1,3,4-oxdiazole moity were designed and synthesized based on the bioisosterism and coordination of active substructure theories. The structures of new compounds were characterized by 1H NMR, 13C NMR and HRMS spectra. The bioassay indicated that the fungicidal activities of some compounds against Sclerotinia sclerotiorum reached the activity of azoxystrobin as the control and some compounds against Rhizotonia cerealis in vitro were more effective than the same control. Their structure-activity relationship was discussed. Methyl (E)-2-(2-((4-(1,3,4-oxadiazol-2-yl)phenoxy)methyl)phenyl)-3-methoxyacrylate (IIa) and methyl (E)-2-(2-((4-(5-ethyl-1,3,4-oxadiazol-2-yl)phe-noxy)methyl)phenyl)-3-methoxyacrylate (IIc) could be considered as leading compounds for further investigation.
Key words: 1,3,4-oxdiazole; strobilurin; in vitro; fungicidal activity
Liu Yang , Liu Ming , Chen Minggui , Wu Changchun , Hua Xuewen , Zhou Sha , Wang Baolei , Li Zhengming . Design, Synthesis and Bioactivities of Novel Strobilurin Derivatives Containing 1,3,4-Oxadiazole Moity[J]. Chinese Journal of Organic Chemistry, 2017 , 37(2) : 403 -410 . DOI: 10.6023/cjoc201607029
[1] Chai, B.-S.; Wang, S.-Y.; Yu, W.-Q.; Li, H.-C.; Song, C.-J.; Xu, Y.; Liu, C.-L.; Chang, J.-B. Bioorg. Med. Chem. Lett. 2013, 23, 3505.
[2] Huang, W.; Zhao, P.-L.; Liu, C.-L.; Chen, Q.; Liu, Z.-M.; Yang, G.-F. J. Agric. Food Chem. 2007, 55, 3004.
[3] Sauter, H.; Steglich, W.; Anke, T. Angew. Chem., Int. Ed. 1999, 38, 1328.
[4] Chen, H.-S.; Li, Z.-M.; Han, Y.-F. J. Agric. Food Chem. 2000, 48, 5312.
[5] Singh, N.; Sangwan, N. K.; Dhindsa, K. S. Pest Manage. Sci. 2000, 56, 284.
[6] Tanitame, A.; Oyamada, Y.; Ofuji, K.; Fujimoto, M.; Iwai, N.; Hiyama, Y.; Suzuki, K.; Ito, H.; Terauchi, H.; Kawasaki, M. J. Med Chem. 2004, 47, 3693.
[7] Bekhit, A. A.; Ashour, H.; Guemei, A. A. Arch. Pharm. 2005, 338, 167.
[8] Fan, Z.-J.; Yang, Z.-K.; Zhang, H.-K.; Mi, N.; Wang, H.; Cai, F.; Zuo, X.; Zheng, Q.-X.; Song, H.-B. J. Agric. Food Chem. 2009, 58, 2630.
[9] Dickinson, R. P.; Bell, A. S.; Hitchcock, C. A.; Narayanaswami, S.; Ray, S. J.; Richardson, K.; Troke, P. F. Bioorg. Med. Chem. Lett. 1996, 6, 2031.
[10] Onodera, J.; Sato, S.; Kumazawa, S.; Ito, A.; Saishoji, S.; Niizeki, Y. JP 96127568, 1996 [Chem. Abstr. 1996, 125, 142713].
[11] Li, Y.; Zhang, H.-Q.; Liu, J.; Yang, X.-P.; Liu, Z.-J. J. Agric. Food Chem. 2006, 54, 3636.
[12] Park, H.-J.; Lee, K.; Park, S.-J.; Ahn, B.; Lee, J.-C.; Cho, H.; Lee, K.-I. Bioorg. Med. Chem. Lett. 2005, 15, 3307.
[13] Naito, H.; Ohsuki, S.; Atsumi, R.; Minami, M.; Mochizuki, M.; Hirotani, K.; Kumazawa, E.; Ejima, A. Chem. Pharm. Bull. 2005, 53, 153.
[14] Tu, S.; Xie, Y.-Q.; Gui, S.-Z.; Ye, L.-Y.; Huang, Z.-L.; Huang, Y.-B.; Che, L.-M. Bioorg. Med. Chem. Lett. 2014, 24, 2173.
[15] Liu, J.-C; Wang, W.-D; He, H.-W. Chin. J. Org. Chem. 2014, 37, 1447 (in Chinese).(刘建超, 王卫东, 贺红武, 有机化学, 2014, 37, 1447.)
[16] Xu, G.-F.; Song, B.-A.; Bhadury, P. S.; Yang, S.; Zhang, P.-Q.; Jin, L.-H.; Xue, W.; Hu, D.-Y.; Lu, P. Bioorg. Med. Chem. 2007, 15, 3768.
[17] Rajput, A.; Kankhare, A. Pharma Chem. 2015, 7, 143.
[18] Wu, X.-L.; Zhu, C.-F.; Lv, Z.-D.; Wei, C.-S.; Liao, C.-C. Chin. J. Org. Chem. 2011, 31, 824 (in Chinese).(武现丽, 朱春风, 吕志丹, 魏成事, 廖新成, 有机化学, 2011, 31, 824.)
[19] Luo, X.-Q. M.S. Thesis, Guizhou University, Guiyang, 2008 (in Chinese).(罗小琼, 硕士论文, 贵州大学, 贵阳, 2008.)
[20] Chen, M. M.S. Thesis, Nanjing Agriculture University, Nanjing, 2011 (in Chinese).(陈敏, 硕士论文, 南京农业大学, 南京, 2011.)
[21] Marty, M. S. R.; Rnaelingam, T. Indian J. Chem., Sect. B 1988, 27B. 293.
[22] Paehhmaia, V. L.; Parikh, A. R. J. Indian Chem. Soc. 1988, 65. 357.
[23] Li, Q.-Z; Song, B.-A; Chen, J. Pesticide 2005, 44. 538 (in Chi-nese).
(李黔柱, 宋宝安, 陈江, 刘杰, 杨松, 胡德禹, 金林红, 农药, 2005, 44. 538.)
[24] Jha, K. K.; Samad, A.; Kumar, Y.; Shaharyar, M.; Khosa, R. L.; Jain, J.; Kumar, V.; Singh, P. Eur. J. Med. Chem. 2010, 45, 4963.
[25] Basoglu, S.; Yolal, M.; Demirci, S.; Demirbas, N.; Bektas, H.; Karaoglu, S. Acta Pol. Pharm. 2013, 70, 229.
[26] Liu, X.-H.; Chen, P.-Q.; Wang, B.-L.; Dong, W.-L. Chem. Biol. Drug Des. 2010, 75, 228.
[27] Ahmad, R.; Iqbal, R.; Akhtar, H.; Haq, Z.; Duddeck, H.; Stefaniak, L.; Sitkowski, J. Nucleosides, Nucleotides Nucleic Acids 2001, 20, 1671.
[28] Patel, M.; Modi, N.; Raval, J.; Menon, S. Org. Biomol. Chem. 2012, 10, 1785.
[29] Rao, M.; Rajurkar, V. Asian J. Chem. 2011, 23, 2648.
[30] S?awiński, J.; Pogorzelska, A.; ?o?nowska, B.; Bro?ewicz, K.; Vullo, D.; Supuran, C. T. Eur. J. Med. Chem. 2014, 82, 47.
[31] Sadek, B.; Fahelelbom, K. Molecules 2011, 16, 4339.
[32] Arnold, L. D.; Coe, J. W.; Kaneko, T.; Moyer, M. P. US 6130217, 2000 [Chem. Abstr. 2000, 133, 296449].
[33] Yildirim, N.; Bekircan, O. J. Chem. Res. 2013, 37, 160.
[34] Cliff, G. R.; Richards; I. C. EP 378308, 1990 [Chem. Abstr. 1991, 114, 81863].
[35] Chen, Y.-W.; Wan, Y.-Y.; Liu, Q.-X.; Liu, J.-B.; Xiong, L.-X.; Yu, S.-J.; Li, Z.-M. Chin. J. Org. Chem. 2015, 35, 882 (in Chinese).(陈有为, 万莹莹, 刘巧霞, 刘敬波, 熊丽霞, 于淑晶, 李正名, 有机化学, 2015, 35, 882.)
[36] Chen, N.-C. Bioassay Technology for Pestcides, Beijing Agricultural University Press, Beijing, 1991, p. 161 (in Chinese).(陈春年, 农药生物技术测定, 北京农业大学出版社, 北京, 1991, p. 161.)
[37] Li, Y.; Gao, Z.-S.; Li, J.-T.; Liu, F. J. Peanut Sci. 2012, 41, 13 (in Chinese).(李杨, 高志山, 李建涛, 刘峰, 花生学报, 2012, 41, 13.)
/
| 〈 |
|
〉 |