

Chinese Journal of Organic Chemistry >
A Reaction-Based Chemsensor for Hydrogen Sulfide Detection with Fluorescence Enhancement
Received date: 2016-07-20
Revised date: 2016-10-08
Online published: 2016-10-18
Supported by
Project supported by the the National Natural Science Foundation of China(No. 21233011) and the State Key Development Program for Basic Research of China(973 Program, Nos. 2013CB834703, 2013CB834505).
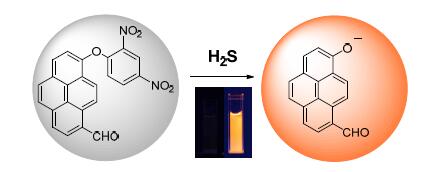
A reaction-based probe 8-(2,4-dinitrophenoxy) pyrene-1-carbaldehyde(PCNP) for H2S detection was designed and synthesized by utilizing 8-hydroxypyrene-1-carbaldehyde and dinitrophenyl ether as the reporting chromophore and the recognition unit,respectively.The probe was dissolved in phosphate buffer saline(PBS) with cetyltrimethyl ammonium bromide(CTAB),and the sensing ability toward H2S was investigated with steady-state spectroscopy.The emission of PCNP was quenched by photoinduced electron transfer without H2S.The fluorescence of PCNP was boosted after thiolysis reaction in the presence of H2S,showing orange red emission.The fluorescence response was rapid and reached a maximum enhancement of about 260 times in the presence of 0.1 mmol·L-1 H2S,giving a reaction rate constant of 0.20 min-1.The limit of detection of PCNP is estimated to be 0.10 μmol·L-1.The results demonstrate that PCNP is capable of detecting H2S rapidly,sensitively and selectively.
Zhou Chan , Qiu Bo , Zeng Yi , Chen Jinping , Yu Tianjun , Li Yi . A Reaction-Based Chemsensor for Hydrogen Sulfide Detection with Fluorescence Enhancement[J]. Chinese Journal of Organic Chemistry, 2017 , 37(1) : 92 -96 . DOI: 10.6023/cjoc201607034
[1] Lin, V. S.; Chen, W.; Xian, M.; Chang, C. J. Chem. Soc. Rev. 2015, 44, 4596.
[2] Kimura, H. Antioxid. Redox Signaling 2014, 20, 783.
[3] Paul, B. D.; Sbodio, J. I.; Xu, R. S.; Vandiver, M. S.; Cha, J. Y.; Snowman, A. M.; Snyder, S. H. Nature 2014, 509, 96.
[4] Hu, L. F.; Lu, M.; Tiong, C. X.; Dawe, G. S.; Hu, G.; Bian, J. S. Aging Cell 2010, 9, 135.
[5] Giuliani, D.; Ottani, A.; Zaffe, D.; Galantucci, M.; Strinati, F.; Lodi, R.; Guarini, S. Neurobiol. Learn. Mem. 2013, 104, 82.
[6] Gao, M.; Yu, F. B.; Chen, L. X. Prog. Chem. 2014, 26, 1065(in Chinese).(高敏, 于法标, 陈令新, 化学进展, 2014, 26, 1065.)
[7] Qian, Y.; Karpus, J.; Kabil, O.; Zhang, S. Y.; Zhu, H. L.; Banerjee, R.; Zhao, J.; He, C. Nat. Commun. 2011, 2, 495.
[8] Peng, B.; Chen, W.; Liu, C. R.; Rosser, E. W.; Pacheco, A.; Zhao, Y.; Aguilar, H. C.; Xian, M. Chem.-Eur. J. 2014, 20, 1010.
[9] Yu, H. B.; Li, H. L.; Zhang, X. F.; Xiao, Y.; Fang, P. J.; Lv, C. J.; Hou, W. Acta Chim. Sinica 2015, 73, 450(in Chinese).(于海波, 李红玲, 张新福, 肖义, 方沛菊, 吕春娇, 侯伟, 化学学报, 2015, 73, 450.)
[10] Zhao, C. C.; Zhang, X. L.; Li, K. B.; Zhu, S. J.; Guo, Z. Q.; Zhang, L. L.; Wang, F. Y.; Fei, Q.; Luo, S. H.; Shi, P.; Tian, H.; Zhu, W. H. J. Am. Chem. Soc. 2015, 137, 8490.
[11] Lippert, A. R.; New, E. J.; Chang, C. J. J. Am. Chem. Soc. 2011, 133, 10078.
[12] Zheng, Y.; Zhao, M.; Qiao, Q. L.; Liu, H. Y.; Lang, H. J.; Xu, Z. C. Dyes Pigm. 2013, 98, 367.
[13] Sun, W.; Fan, J. L.; Hu, C.; Cao, J. F.; Zhang, H.; Xiong, X. Q.; Wang, J. Y.; Cui, S.; Sun, S. G.; Peng, X. J. Chem. Commun. 2013, 49, 3890.
[14] Fan, F. L.; Jing, J. Q.; Chen, X. M. Chin. J. Org. Chem. 2014, 34, 2178(in Chinese).(范方禄, 靖金球, 陈雪梅, 有机化学, 2014, 34, 2178.)
[15] Sasakura, K.; Hanaoka, K.; Shibuya, N.; Mikami, Y.; Kimura, Y.; Komatsu, T.; Ueno, T.; Terai, T.; Kimura, H.; Naganot, T. J. Am. Chem. Soc. 2011, 133, 18003.
[16] Qu, X. Y.; Li, C. J.; Chen, H. C.; Mack, J.; Guo, Z. J.; Shen, Z. Chem. Commun. 2013, 49, 7510.
[17] Liu, J.; Guo, X. D.; Hu, R.; Liu, X. Y.; Wang, S. Q.; Li, S. Y.; Li, Y.; Yang, G. Q. Anal. Chem. 2016, 88, 1052.
[18] Fridkin, M.; Hazum, E.; Tauberfinkelstein, M.; Shaltiel, S. Arch. Biochem. Biophys. 1977, 178, 517.
[19] Liu, T. Y.; Xu, Z. C.; Spring, D. R.; Cui, J. N. Org. Lett. 2013, 15, 2310.
[20] Yuan, L.; Zuo, Q. P. Sens. Actuators, B 2014, 196, 151.
[21] Liu, Y.; Feng, G. Q. Org. Biomol. Chem. 2014, 12, 438.
[22] Cao, L. X.; Li, X. Y.; Wang, S. Q.; Li, S. Y.; Li, Y.; Yang, G. Q. Chem. Commun. 2014, 50, 8787.
[23] Qiu, B.; Zeng, Y.; Cao, L. X.; Hu, R.; Zhang, X. H.; Yu, T. J.; Chen, J. P.; Yang, G. Q.; Li, Y. RSC Adv. 2016, 6, 49158.
/
| 〈 |
|
〉 |