

Chinese Journal of Organic Chemistry >
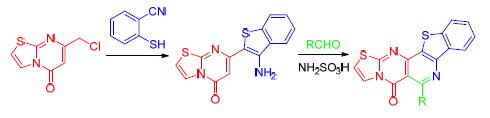
An Efficient Synthesis of Benzothieno-[3',2': 2,3]pyrido[4,5-d]thiazolo[3,2-a]pyrimidin-5-ones
Received date: 2016-09-29
Revised date: 2016-11-07
Online published: 2016-11-22
Supported by
Project supported by the Innovation Team Project of Liaoning Province Education Department (No. 2015001).
A series of novel benzothieno[3',2':2,3]pyrido[4,5-d]thiazolo[3,2-a]pyrimidinone derivatives are prepared via sulfamic acid-catalyzed Pictet-Spengler cyclization of 7-(3-amino-5-phenylaminothiazolo-2-yl) thiazolo[3,2-a]pyrimidin-5-one, which in turn were obtained from the Thorpe-Ziegler isomerization of 7-(chloromethyl)thiazolo[3,2-a]pyrimidin-5-one with 2-mercaptobenzonitrile. The method was characterized with raw material easy to get, mild reaction conditions, good yields and a facile and efficient synthetic method of novel fused pyrimidines.
Wang Dong , Wang Daolin , Qian Jianhua . An Efficient Synthesis of Benzothieno-[3',2': 2,3]pyrido[4,5-d]thiazolo[3,2-a]pyrimidin-5-ones[J]. Chinese Journal of Organic Chemistry, 2017 , 37(3) : 698 -703 . DOI: 10.6023/cjoc201609031
[1] Dinakaran, V. S.; Bomma, B.; Srinivasan, K. K. Pharma Chem. 2012, 4, 255.
[2] Flefel, E. E.; M. Salama, A.; El-Shahat, M. Phosphorus, Sulfur Silicon Relat. Elem. 2007, 182, 1739.
[3] Tozkoparan, B.; Ertan, M.; Kelicen, P.; Demirdamar, R. Farmaco 1999, 54, 588.
[4] Mohamed, S. F.; Flefel, E. M.; Amr, A. E.-G. E.; Abd El-Shafy, D. N. Eur. J. Med. Chem. 2010, 45, 1494.
[5] Alam, O.; Khan, S. A.; Siddiqui, N.; Ahsan, W. Med. Chem. Res. 2010, 19, 1245.
[6] (a) Kulakov, I. V. Chem. Heterocycl. Compd. 2009, 45, 1019.
(b) Abbas, A. E.; Mahdieh, Z.; Ali, R. F.; Azizollah, H. Tetrahedron Lett. 2012, 53, 1351.
(c) Abd El-Galil, E. A.; Maigali, S. S.; Abdulla, M. M. Monatsh. Chem. 2008, 139, 1409.
[7] (a) Huang, J.; Luo, H.; Wang, L.; Guo, Y.; Zhang, W.; Chen, H.; Zhu, M.; Liu Y.; Yu, G. Org. Lett. 2012, 14, 3300.
(b) Ni, Y.; Nakajima, K.; Kanno K.; Takahashi, T. Org. Lett. 2009, 11, 3702.
[8] Romagnoli, R.; Baraldi, P. G.; Carrion, M. D.; Cara, C. L.; Preti, D.; Fruttarolo, F.; Pavani, M. G.; Tabrizi, M. A.; Tolomeo, M.; Grimaudo, S.; Cristina, A. D.; Balzarini, J.; Hadfield, J. A.; Brancale, A.; Hamel, E. J. Med. Chem. 2007, 50, 2273.
[9] Chonan, T.; Wakasugi, D.; Yamamoto, D.; Yashiro, M.; Oi, T.; Tanaka, H.; Ohoka-Sugita, A.; Io, F.; Koretsune H.; Hiratate, A. Bioorg. Med. Chem. 2011, 19, 1580.
[10] Berrade, L.; Aisa, B.; Ramirez, M. J.; Galiano, S.; Guccione, S.; Moltzau, L. R.; Levy, F. O.; Nicoletti, F.; Battaglia, G.; Molinaro, G.; Aldana, I.; Monge, A.; Perez-Silanes, S. J. Med. Chem. 2011, 54, 3086.
[11] Lee, K. C.; Moon, B. S.; Lee, J. H.; Chung, K. H.; Katzenellenbogen, J. A.; Chi, D. Y. Bioorg. Med. Chem. 2003, 11, 3649.
[12] Vogel, V. G.; Costantino, J. P.; Wickerham, D. L.; Cronin, W. M.; Cecchini, R. S.; Atkins, J. N.; Bevers, T. B.; Fehrenbacher, L.; Pajon, E. R.; Wade, J. L.; Robidoux, A.; Margolese, R. G.; James, J.; Lippman, S. M.; Runowicz, C. D.; Ganz, P. A.; Reis, S. E.; McCaskill-Stevens, W.; Ford, L. G.; Jordan V. C.; Wolmark, N. 2006, 295, 2727.
[13] Lu, P.; Schrag, M. L.; Slaughter, D. E.; Raab, C. E.; Shou, M.; Rodrigues, A. D. Drug Metab. Dispos. 2003, 31, 1352.
[14] Croxtall, J. D.; Plosker, G. L. Drugs 2009, 69, 339.
[15] Pictet, A.; Spengler, T. T. Ber. 1911, 44, 2030.
[16] Chrzanowska, M.; Rozwadowska, M. D. Chem. Rev. 2004, 104, 3341.
[17] (a) Kundu, B.; Sawant, D.; Partani, P.; Kesarwani, A. P. J. Org. Chem. 2005, 70, 4889.
(b) Duggineni, S.; Sawant, D.; Saha, B.; Kundu, B. Tetrahedron 2006, 62, 3228.
(c) Saha, B.; Sharma, D.; Sawant, D.; Kundu, B. Tetrahedron 2008, 64, 8676.
[18] Kundu, B.; Agarwal, P. K.; Sharma, S. K.; Sawant, D.; Man-dadapu, A. K.; Saifuddin, M.; Gupta, S. Curr. Org. Synth. 2012, 9, 357.
[19] (a) Wang, D. L.; Wang, D.; Yan, L.; Pan, G. Y.; Yang, J. N. Heterocycles 2016, 92, 552.
(b) Wang, D. L.; Wang, D.; Yan, L.; Pan, G. Y.; Yang, J. N. Chin. Chem. Lett. 2016, 27, 953.
[20] (a) Litvinov, V. P.; Dotsenko, V. V.; Krivokolysko, S. G. Russ. Chem. Bull. 2005, 54, 864.
(b) Litvinov, V. P.; Dotsenko, V. V.; Krivokolysko, S. G. In Advances in Heterocyclic Chemistry, Ed.:Katritzky, A. R., Academic, New York, 2007, 93, 117.
[21] Djekou, S.; Gellisa, A.; Vanelle, P.; El-Kashef, H. J. Heterocycl. Chem. 2006, 43, 1225.
[22] Zhou J. F.; Gong, G. X.; An, L. T.; Sun, X. J.; Zhu. F. X. Chin. J. Org. Chem. 2009, 29, 1988 (in Chinese).(周建峰, 贡桂霞, 安礼涛, 孙小军, 朱凤霞, 有机化学, 2009, 29, 1988.)
/
| 〈 |
|
〉 |