

Chinese Journal of Organic Chemistry >
Synthesis and Antitumor Activity of N-[4-(t-Butyl)-5-benzylthiazol-2-yl]amininoacetamides
Received date: 2016-10-12
Revised date: 2016-11-16
Online published: 2016-11-29
Supported by
Project supported by the National Natural Science Foundation of China (No. 21442014).
The antitumor activity research of N-(thiazol-2-yl) amide derivatives has been the focus of scholars. Physiologi-cally active substances containing amines widely exist in nature. A series of novel N-(4-(t-butyl)-5-benzylthiazol-2-yl) aminoacetamides were synthesized, and their structures were confirmed by 1H NMR, 13C NMR and elemental analysis. These compounds were evaluated for their in vitro anticancer activity on A549, Hela and MCF-7 cells. Most of the investigated compounds exhibited broad-spectrum antitumor activity. Compound 1t displayed significant activity against Hela cancer cells with IC50 value of (6.4±2.2) μmol/L. The AO/EB staining and cell cycle experiments were carried out on the preferred com-pound 1t. The result showed that compound 1t could significantly induce apoptosis of tumor cells and arrest the Hela cells in the S phase.
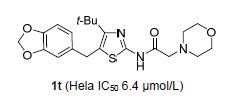
Key words: thiazoleacetamide; morpholine; synthesis; antitumor activity
Tang Yuting , Ding Na , Wu Zhilin , Ye Jiao , Shen Kun , Hu Aixi . Synthesis and Antitumor Activity of N-[4-(t-Butyl)-5-benzylthiazol-2-yl]amininoacetamides[J]. Chinese Journal of Organic Chemistry, 2017 , 37(3) : 675 -682 . DOI: 10.6023/cjoc201610018
[1] Pokhodylo, N.; Shyyka, O.; Matiychuk, V. Med. Chem. Res. 2014, 23, 2426.
[2] Lee, Y. S. E.; Chuang, S. H.; Huang, L. Y. L.; Lai, C. L.; Lin, Y. H.; Yang, J. Y.; Liu, C. W.; Yang, S. C.; Lin, H. S.; Chang, C. C.; Lai, J. Y.; Jian, P. S.; Lam, K.; Chang, J. M.; Lau, J. Y. N.; Huang, J. J. J. Med. Chem. 2014, 57, 4098.
[3] Gorczynski, M. J.; Leal, R. M.; Mooberry, S. L.; Bushweller, J. H.; Brown, M. L. Bioorg. Med. Chem. 2004, 12, 1029.
[4] Jiang, F. C.; Cheng, C. Y. Acta Pharm. Sin. 2006, 41, 727 (in Chinese).(姜凤超, 成冲云, 药学学报, 2006, 41, 727.)
[5] Bjoern, E.; Guido, K.; Christian, H. CN 101277692, 2007[Chem. Abstr. 2007, 146, 163101].
[6] Chen, Y.; Wang, H.; Dinesh, A.; Zhou, C. H. Chin. J. Org. Chem. 2016, 36, 1 (in Chinese).(程宇, 王辉, Dinesh Addla, 周成合, 有机化学, 2016, 36, 1.)
[7] Liao, Q. H.; Lin, S.; Deng, R. H.; Huang, Z. Q.; Deng, K. Y.; Yan, Z. H. Chin. J. Org. Chem. 2015, 35, 1923 (in Chinese).(廖启华, 林森, 邓瑞红, 黄志强, 邓柯玉, 严兆华, 有机化学, 2015, 35, 1923.)
[8] (a) Hu, A. X.; Huo, S. F.; Xia, S.; Li, W.; Ye, J.; Peng, J. M.; Xiang, J. N. CN 102070556, 2011[Chem. Abstr. 2011, 154, 615156].
(b) Hu, A. X.; Li, W.; Xia, S.; Ye, J.; Huo, S. F.; Zou, S. S.; Peng, J. M.; Xiang, J. N. CN 102319244, 2012[Chem. Abstr. 2012, 156, 194844].
[9] Makam, P.; Kannan, T. Eur. J. Med. Chem. 2014, 87, 643.
[10] Turan-Zitouni, G.; Özdemir, A.; Kaplanc?kli, Z. A. Phosphorus, Sulfur, Silicon Relat. Elem. 2011, 186, 233.
[11] Kouatly, O.; Geronikaki, A.; Kamoutsis, C.; Hadjipavlou-Litina, D.; Eleftheriou, P. Eur. J. Med. Chem. 2009, 44, 1198.
[12] Siddiqui, N.; Ahsan, W. Eur. J. Med. Chem. 2010, 45, 1536.
[13] Iino, T.; Tsukahara, D.; Kamata, K.; Sasaki, K.; Ohyama, S.; Hosaka, H.; Hasegawa, T.; Chiba, M.; Nagata, Y.; Eiki, J.; Nishimura, T. Bioorg. Med. Chem. 2009, 17, 2733.
[14] Abdel-Wahab, B. F.; Mohamed, S. F.; Amr, A. E. G. E.; Abdalla, M. Monatsh. Chem. 2008, 139, 1083.
[15] Rostom, S. A. F.; Faidallah, H. M.; Radwan, M. F.; Badr, M. H. Eur. J. Med. Chem. 2014, 76, 170.
[16] Gurdal, E. E.; Durmaz, I.; Cetin-Atalay, R.; Yarim, M. J. Enzyme Inhib. Med. Chem. 2015, 30, 649.
[17] Cui, J. G.; Zhao, D. D.; He, D. M.; Huang, Y. M.; Liu, Z. P.; Lin, Q. F.; Shi, H. X.; Gan, C. F. Chin. J. Org. Chem. 2016, 36, 630 (in Chinese).(崔建国, 赵丹丹, 何冬梅, 黄燕敏, 刘志平, 林啟福, 石海信, 甘春芳, 有机化学, 2016, 36, 630.)
[18] (a) Hu, A. X.; Peng, J. M.; Fang, Y. L.; Shen, K.; Li, W.; Yan, X. W. CN 103333132, 2013[Chem. Abstr. 2013, 158, 359731].
(b) Hu, A. X.; Peng, J. M.; Shen, K.; Li, W.; Yan, X. W.; Fang, Y. L. CN 103601697, 2013[Chem. Abstr. 2013, 158, 446959].
[19] Peng, J. M. Ph.D. Dissertation, Hunan University, Changsha, 2013, (in Chinese).(彭俊梅, 博士论文, 湖南大学, 长沙, 2013.)
[20] Wu, Z. L.; Fang, Y. L.; Tang, Y. T.; Xiao, M. W.; Ye, J.; Li, G. X. A.; Hu, X. Med. Chem. Commun. 2016, 7, 1768.
[21] Peng, J. M.; Li, W.; Shen, K.; Huo, S. F.; Ye, J.; Hu, A. X. Chem. J. Chin. Univ. 2013, 34, 1646 (in Chinese).(彭俊梅, 李婉, 申坤, 霍素芳, 叶姣, 胡艾希, 高等学校化学学报, 2013, 34, 1646.)
/
| 〈 |
|
〉 |