

Chinese Journal of Organic Chemistry >
Palladium-Catalyzed C-H Alkoxycarbonylation of Caffeines: Synthesis of 8-Ester-substituted Caffeines
Received date: 2016-10-17
Revised date: 2016-12-08
Online published: 2016-12-12
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 2110322, 6152010615) and the Foundation of Fujian Educational Committee (No. JK2014010).
Carbonyl-substituted caffeine derivatives have attracted much attention due to their potent pharmaceutical activity and interesting fluorescent properties. An efficient synthesis of 8-ester-substituted caffeines through palladium-catalyzed C-H alkoxycarbonylation was developed. The reaction was carried out in the presence of PdCl2(PPh3)2 and Cu(OAc)2 under 101 kPa CO atmosphere in 1,4-dioxane, providing diversified 8-ester-substituted caffeines in reasonable to good yields. The approach was characterized by using atmospheric pressure of carbon monoxide and broad functional group tolerance.
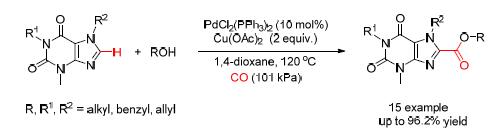
Key words: palladium; C-H bond acitivation; carbonylation; caffeine; carbon monoxide
Su Lü, Xiao Hanbing, Yuan Yumeng, Zhang Xiaofeng, Lin Shen, Huang Qiufeng . Palladium-Catalyzed C-H Alkoxycarbonylation of Caffeines: Synthesis of 8-Ester-substituted Caffeines[J]. Chinese Journal of Organic Chemistry, 2017 , 37(3) : 630 -635 . DOI: 10.6023/cjoc201610028
[1] (a) Quintero-Duque, S.; Dyballa, K. M.; Fleischer, I. Tetrahedron Lett. 2015, 56, 2634.
(b) Wu, X.-F.; Fang, X.; Wu, L.; Jackstell, R.; Neumann, H.; Beller, M. Acc. Chem. Res. 2014, 47, 1041.
(c) Sumino, S.; Fusano, A.; Fukuyama, T.; Ryu, I. Acc. Chem. Res. 2014, 47, 1563.
(d) Barnard, C. F. J. Organometallics 2008, 27, 5402.
(e) Wu, X.-F.; Neumann, H.; Beller, M. Chem. Rev. 2013, 113, 1.
(f) Omae, I. Coordin. Chem. Rev. 2011, 255, 139.
[2] (a) Schoenberg, A.; Bartoletti, I.; Heck, R. F. J. Org. Chem. 1974, 39, 3318.
(b) Schoenberg, A.; Heck, R. F. J. Org. Chem. 1974, 39, 3327.
(c) Schoenberg, A.; Heck, R. F. J. Am. Chem. Soc. 1974, 96, 7761.
[3] (a) Wu, L.; Fang, X.; Liu, Q.; Jackstell, R.; Beller, M.; Wu, X.-F. ACS Catal. 2014, 4, 2977.
(b) Sumino, S.; Fusano, A.; Fukuyama, T.; Ryu, I. Acc. Chem. Res. 2014, 47, 1563.
(c) Wu, X.-F.; Neumann, H.; Beller, M. Chem. Soc. Rev. 2011, 40, 4986.
(d) Brennführer, A.; Neumann, H.; Beller, M. Angew. Chem., Int. Ed. 2009, 48, 4114.
(e) Sargent, B. T.; Alexanian, E. J. J. Am. Chem. Soc. 2016, 138, 7520.
[4] (a) Liu, B.; Hu, F.; Shi, B.-F. ACS Catal. 2015, 5, 1863.
(b) Wu, X.-F.; Neumann, H.; Beller, M. ChemSusChem 2013, 6, 229.
(c) Liu, Q.; Zhang, H.; Lei, A. Angew. Chem., Int. Ed. 2011, 50, 10788.
(d) Gadge, S. T.; Bhanage, B. M. RSC Adv. 2014, 4, 10367.
(e) Fenner, S.; Ackermann, L. Green Chem. 2016, 18, 3804.
(f) Boogaerts, I. I. F.; Nolan, S. P. J. Am. Chem. Soc. 2010, 132, 8858.
[5] (a) Müller, C. E.; Jacobson, K. A. Biochim. Biophys. Acta 2011, 1808, 1290.
(b) Stydom, B.; Bergh, J. J.; Petzer, J. P. Eur. J. Med. Chem. 2011, 46, 3474.
(c) Van der Walt, M. M.; Terre'Blanche, G.; Petzer, A.; Lourens, A. C. U.; Petzer, J. P. Bioorg. Chem. 2013, 49, 49.
(d) Rivara, S.; Piersanti, G.; Bartoccini, F.; Diamantini, G.; Pala, D.; Riccioni, T.; Stasi, M. A.; Cabri, W.; Borsini, F.; Mor, M.; Tarzia, G.; Minetti, P. J. Med. Chem. 2013, 56, 1247.
(e) Baraldi, P. G.; Baraldi, S.; Saponaro, G.; Preti, D.; Romagnoli, R.; Piccagli, L.; Cavalli, A.; Recanatini, M.; Moorman, A. R.; Zaid, A. N.; Varani, K.; Borea, P. A.; Tabrizi, M. J. J. Med. Chem. 2012, 55, 797.
[6] Uchil, V.; Seo, B.; Nair, V. J. Org. Chem. 2007, 72, 8577.
[7] (a) Zhang, H.; Zhou, L.; Zhu, Z.; Yang, C. Chem. Eur. J. 2016, 22, 9886.
(b) Kocaoglu, O.; Carlson, E. E. Nat. Chem. Biol. 2016, 12, 472.
(c) Rice, D. R.; Clear, K. J.; Smith, B. D. Chem. Commun. 2016, 52, 8787.
[8] (a) Greco, N. J.; Tor, Y. Tetrahedron 2007, 63, 3515.
(b) Kim, D.; Lee, J. H.; Hong, S.-S.; Hong, S. Org. Lett. 2010, 12, 1212.
(c) Zhao, D.; Wang, W.; Yang, F.; Lan, J.; Yang, L.; Gao, G.; You, J. Angew. Chem., Int. Ed. 2009, 48, 3296.
(d) Huang, Y.; Song, F.; Wang, Z.; Xi, P.; Wu, N.; Wang, Z.; Lan, J.; You, J. Chem. Commun. 2012, 48, 2864.
[9] Heer, J. P.; Smith, I. E. D. WO 2007017265, 2007[Chem. Abstr. 2007, 146, 251660].
[10] (a) Neufeldt, S. R.; Sanford, M. S. Acc. Chem. Res. 2012, 45, 936.
(b) Li, H.; Shi, Z. Prog. Chem. 2010, 22, 1414 (in Chinese).(李湖, 施章杰, 化学进展, 2010, 22, 1414).
(c) Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147.
(d) Liu, C.; Yuan, J.; Gao, M.; Tang, S.; Li, W.; Shi, R.; Lei, A. Chem. Rev. 2015, 115, 12138.
(e) Chen, Z.; Wang, B.; Zhang, J.; Yu, W.; Liu, Z.; Zhang, Y. Org. Chem. Front. 2015, 2, 1107.
(f) Gang, F.; Xu, G.; Dong, T.; Yang, L.; Du, Z. Chin. J. Org. Chem. 2015, 35, 1428 (in Chinese).(刚芳莉, 徐关利, 董涛生, 杨丽, 杜正银, 有机化学, 2015, 35, 1428.)
[11] (a) Lang, R.; Shi, L.; Li, D.; Xia, C.; Li, F. Org. Lett. 2012, 14, 4130.
(b) Chen, M.; Ren, Z.-H.; Wang, Y.-Y.; Guan, Z.-H. J. Org. Chem. 2015, 80, 1258.
(c) Li, X.; Li, X.; Jiao, N. J. Am. Chem. Soc. 2015, 137, 9246.
(d) Yoo, E. J.; Wasa, M, Yu, J.-Q. J. Am. Chem. Soc. 2010, 132, 17378.
(e) Lian, Z.; Friis, S. D.; Skrydstrup, T. Chem. Commun. 2015, 51, 1870.
(f) Liu, B.; Hu, F.; Shi, B.-F. ACS Catal. 2015, 5, 1863.
[12] Chen, M.; Ren, Z.-H.; Wang, R.-Y.; Guan, Z.-H. Angew. Chem., Int. Ed. 2013, 52, 14196.
[13] Lang, R.; Wu, J.; Shi, L.; Xia, C.; Li, F. Chem. Commun. 2011, 47. 12553.
[14] Zhang, H.; Liu, D.; Chen, C.; Liu, C.; Lei, A. Chem. Eur. J. 2011, 17, 9581.
[15] (a) Malakar, C. C.; Schmidt, D.; Conrad, J.; Beifuss, U. Org. Lett. 2011, 13, 1378.
(b) Daly, J. W.; Padgett, W. L.; Shamim, M. T. J. Med. Chem. 1986, 29, 1305.
/
| 〈 |
|
〉 |