

Chinese Journal of Organic Chemistry >
[4+4] Photodimerization of Anthracene Derivatives: Recent Synthetic Advances and Applications
Received date: 2016-11-02
Revised date: 2016-12-06
Online published: 2016-12-12
Supported by
Project supported by the "Thousand Youth Talents Plan", the National Natural Science Foundation of China (No. 21672227), the Chinese Academy of Sciences (No. XDB17030200) and the China Postdoctoral Science Foundation (No. 2016M601140).
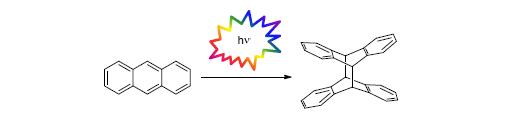
The[4+4] photodimerizations of anthracene and its derivatives have been extensively studied for over a century. This classic photochemical reaction features broad substrate scope, user-friendly operation, and controllable reversibility. However, the synthetic applications of anthracene photodimerizations have been underdeveloped thus far, largely because of stereoisomer separation and solubility problems of the dianthracene products. Considering that the dianthracene products display unique molecular rigidity and geometry, further studies to improve the synthetic utility of anthracene photodimerizations should be warranted. This review summarizes the representative works of anthracene photodimerizations since the beginning of the 21st century, highlighting the synthetic advances to improve the reaction regio- and enantio-selectivity, as well as the synthetic applications toward functional organic molecules and molecular machines.
Liu Weigang , Guo Lifeng , Fan Yangyang , Huang Zeao , Cong Huan . [4+4] Photodimerization of Anthracene Derivatives: Recent Synthetic Advances and Applications[J]. Chinese Journal of Organic Chemistry, 2017 , 37(3) : 543 -554 . DOI: 10.6023/cjoc20161101
[1] Becker, H.-D. Chem. Rev. 1993, 93, 145.
[2] Bouas-Laurent, H.; Castellan, A.; Desvergne,J.-P.; Lapouyade, R. Chem. Soc. Rev. 2000, 29, 43.
[3] Bouas-Laurent, H.; Castellan, A.; Desvergne, J.-P.; Lapouyade, R. Chem. Soc. Rev. 2001, 30, 248.
[4] For selected examples on photodimerizations of heteroaromatics:(a) Zhang, Y.; Wang, L.; Zhang, M.; Fun, H.-K.; Xu, J.-H. Org. Lett. 2004, 6, 4893;
(b) Wang, R.; Yuan, L.; Macartney, D. H. J. Org. Chem. 2006, 71, 1237;
(c) Ihmels, H.; Luo, J. J. Photochem. Photobiol., A 2008, 200, 3.
[5] For selected examples on photodimerizations of naphthalene derivatives:
(a) Tung, C.-H.; Wang, Y.-M. J. Am. Chem. Soc. 1990, 112, 6322.
(b) Lei, L.; Wu, L.-Z.; Wu, X.-L.; Liao, G.-H.; Luo, L.; Zhang, L.-P.; Tung, C.-H.; Ding, K.-L. Tetrahedron Lett. 2006, 47, 4725.
(c) Luo, L.; Cheng, S.-F.; Chen, B.; Tung, C.-H.; Wu, L.-Z; Langmuir 2010, 26, 782.
(d) Xu, H.-X.; Cheng, S.-F.; Yang, X.-J.; Chen, B.; Chen, Y.; Zhang, L.-P.; Wu, L.-Z.; Fang, W.; Tung, C.-H.; Weiss, R. G. J. Org. Chem. 2012, 77, 1685.
[6] For selected examples on photodimerizations of tetracene and pentacene:
(a) Reichwagen, J.; Hopf, H.; Del Guerzo, A.; Desvergne, J.-P.; Bouas-Laurent, H. Org. Lett. 2004, 6, 1899.
(b) Benard, C. P.; 'Geng, Z.; Heuft, M. A.; VanCrey, K.; Fallis, A. G. J. Org. Chem. 2007, 72, 7229.
[7] Takaguchi, Y.; Tajima, T.; Ohta, K.; Motoyoshiya, J.; Aoyama, H. Chem. Lett. 2000, 29, 1388.
[8] Cao, D.; Meier, H. Angew. Chem., Int. Ed. 2001, 40, 186.
[9] Benard, C. P.; Geng, Z.; Heuft, M. A.; VanCrey, K.; Fallis, A. G. J. Org. Chem. 2007, 72, 7229.
[10] Liang, C.-K.; Desvergne, J.-P.; Bassani, D. M. Photochem. Photobiol. Sci. 2014, 13, 316.
[11] Fukuhara, G.; Iida, K.; Kawanami, Y.; Tanaka, H.; Mori, T.; Inoue, Y. J. Am. Chem. Soc. 2015, 137, 15007.
[12] Li, P.; Wong, B. M.; Zakharov, L. N.; Jasti, R. Org. Lett. 2016, 18, 1574.
[13] Tung, C.-H.; Wu, L.-Z.; Zhang, L.-P.; Chen, B. Acc. Chem. Res. 2003, 36, 39.
[14] Schmidt, G. M. J. Pure Appl. Chem. 1971, 27, 647.
[15] Ito, Y.; Fujita, H. J. Org. Chem. 1996, 61, 567.
[16] Ihmels, H.; Leusser, D.; Pfeiffer, M.; Stalke, D. Tetrahedron 2000, 56, 6867.
[17] Horiguchi, M.; Ito, Y. J. Org. Chem. 2006, 71, 3608.
[18] Yamada, S.; Kawamura, C. Org. Lett. 2012, 14, 1572.
[19] Tung, C. H.; Wu, L. Z.; Yuan, Z. Y.; Su, N. J. Am. Chem. Soc. 1998, 120, 11594.
[20] Tung, C.-H., Guang, J.-Q. J. Org. Chem. 1998, 63, 5857.
[21] Wu, D.-Y.; Zhang, L.-P.; Wu, L.-Z.; Wang, B.-J.; Tung, C.-H. Tetrahedron Lett. 2002, 43, 1281.
[22] Wu, D.-Y.; Chen, B.; Fu, X.-G.; Wu, L.-Z.; Zhang, L.-P.; Tung, C.-H. Org. Lett. 2003, 5, 1075.
[23] Gui, J.-C.; Yan, Z.-Q.; Peng, Y.; Yi, J.-G.; Zhou, D.-Y.; Su, D.; Zhong, Z.-H.; Gao, G.-W.; Wu, W.-H.; Yang, C. Chin. Chem. Lett. 2016, 27, 1017.
[24] Marquis, D.; Desvergne, J.-P.; Bouas-Laurent, H. J. Org. Chem. 1995, 60, 7984.
[25] Hiraga, H.; Morozumi, T.; Nakamura, H. Tetrahedron Lett. 2002, 43, 9093.
[26] Rau, H. Chem. Rev. 1983, 83, 535.
[27] Inoue, Y. Chem. Rev. 1992, 92, 741.
[28] Yang, C.; Inoue, Y. Chem. Soc. Rev. 2014, 43, 4123.
[29] Yang, C.; Mori, T.; Origane, Y.; Ko, H. Y.; Selvapalam, N.; Kim, K.; Inoue, Y. J. Am. Chem. Soc. 2008, 130, 8574.
[30] Yang, C.; Ke, C.-F.; Liang, W.; Fukuhara, G.; Mori, T.; Liu, Y.; Inoue, Y. J. Am. Chem. Soc. 2011, 133, 13786.
[31] Yao, J.; Yan, Z.; Ji, J.; Wu, W.; Yang, C.; Nishijima, M.; Fukuhara, G.; Mori, T.; Inoue, Y. J. Am. Chem. Soc. 2014, 136, 6916.
[32] Nishijima, M.; Wada, T.; Mori, T.; Pace, T. C. S.; Bohne, C.; Inoue, Y. J. Am. Chem. Soc. 2007, 129, 3478.
[33] Nishijima, M.; Kato, H.; Fukuhara, G.; Yang, C.; Mori, T.; Maruyama, T.; Otagiri, M.; Inoue, Y. Chem. Commun. 2013, 49, 7433.
[34] Nishijima, M.; Goto, M.; Fujikawa, M.; Yang, C.; Mori, T.; Wada, T.; Inoue, Y. Chem. Commun. 2014, 50, 14082.
[35] Kawanami, Y.; Katsumata, S.-Y.; Nishijima, M.; Fukuhara, G.; Asano, K.; Suzuki, T.; Yang, T.; Nakamura, A.; Mori, T.; Inoue, Y. J. Am. Chem. Soc. 2016, 138, 12187.
[36] Fukuhara, G.; Nakamura, T.; Kawanami, Y.; Yang, C.; Mori, T.; Hiramatsu, H.; Dan-oh, Y.;Tsujimoto, K.; Inoue, Y. Chem. Commun. 2012, 48, 9156.
[37] Fukuhara, G.; Nakamura, T.; Kawanami, Y.; Yang, C.; Mori, T.; Hiramatsu, H.; Dan-oh, Y.; Nishimoto, T.; Tsujimoto, K.; Inoue, Y. J. Org. Chem. 2013, 78, 10996.
[38] Fukuhara, G.; Iida, K.; Kawanami, Y.; Tanaka, H.; Mori, T.; Inoue, Y. J. Am. Chem. Soc. 2015, 137, 15007.
[39] Kohmoto, S.; Ono, Y.; Masu, H.; Yamaguchi, C.; Kishikaw, K.; Yamamoto, M. Org. Lett. 2001, 3, 4153.
[40] Sakamoto, M.; Unosawa, A.; Kobaru, S.; Saito, A.; Mino, T.; Fujita, T. Angew. Chem., Int. Ed. 2005, 44, 5523.
[41] Sakamoto, M.; Unosawa, A.; Kobaru, S.; Hasegawa, Y.; Mino, T.; Kasashima, Y.; Fujita, T.; Chem. Commun. 2007, 1632.
[42] Barth, J. V.; Costantini, G.; Kern, K. Nature 2005, 437, 671.
[43] Kissel, P.; Heijst, J.; Enning, R.; Stemmer, A.; Schlüter, A. D.; Sakamoto, J. Org. Lett. 2010, 12, 2778.
[44] Li, M.; Schlüter, A. D.; Sakamoto, J. J. Am. Chem. Soc. 2012, 134, 11721.
[45] Kissel, P.; Erni, R.; Schweizer, W. B.; Rossell, M. D.; King, B. T.; Bauer, T.; Götzinger, S.; Schlüter, A. D.; Sakamoto, J. Nat. Chem. 2012, 4, 287.
[46] Murray, D. J.; Patterson, D. D.; Payamyar, P.; Bhola, R.; Song, W.; Lackinger, M.; Schlüter, A. D.; King, B. T. J. Am. Chem. Soc. 2015, 137, 3450.
[47] Huang, Z.-A.; Chen, C.; Yang, X.-D.; Fan, X.-B.; Zhou, W.; Tung, C.-H.; Wu, L.-Z.; Cong, H. J. Am. Chem. Soc. 2016, 138, 11144.
[48] Schäfer, C.; Mattay, J. Photochem. Photobiol. Sci. 2004, 3, 331.
[49] Hao, W.; Fang, L.; Helgeson, R. C.; Houk, K. N. Angew. Chem., Int. Ed. 2013, 52, 655.
[50] Molard, Y.; Bassani, D. M.; Desvergne, J.-P.; Horton, P. N.; Hursthouse, M. B.; Tucker, J. H. R. Angew. Chem., Int. Ed. 2005, 44, 1072.
[51] Molard, Y.; Bassani, D. M.; Desvergne, J.-P.; Moran, N.; Tucker, J. H. R. J. Org. Chem. 2006, 71, 8523.
[52] Chih-Kai, L.; Dubacheva, G. V.; Buffeteau, T.; Cavagnat, D.; Hapiot, P.; Fabre, B.; Tucker, J. H. R.; Bassani, D. M. Chem. Eur. J. 2013, 19, 12748.
[53] Hirose, K.; Shiba, Y.; Ishibashi, K.; Doi, Y.; Tobe, Y. Chem. Eur. J. 2008, 14, 3427.
[54] Tron, A.; Thornton, P. J.; Lincheneau, C.; Desvergne, J.-P.; Spencer, N.; Tucker, J. H. R.; McClenaghan, N. D. J. Org. Chem. 2015, 80, 988.
[55] Tron, A.; Jacquot de Rouville, H.-P.; Ducrot, A.;Tucker, J. H. R.; Baroncini, M.; Credi, A.; McClenaghan, N. D. Chem. Commun. 2015, 51, 2810.
[56] Castellano, M.; Ferrando-Soria, J.; Pardo, E.; Julve, M.; Lloret, F.; Mathoniere, C.; Pasan, J.; Ruiz-Perez, C.; Canadillas-Delgado, L.; Ruiz-Garcia, R.; Cano, J. Chem. Commun. 2011, 47, 11035.
[57] Carvalho, C. P.; Dominguez, Z.; Silva, J. P. D.; Pischel, U. Chem. Commun. 2015, 51, 2698.
[58] For selected reviews:
(a) Sieburth, S. M.; Cunard, N. T. Tetrahedron 1996, 52, 6251.
(b) Hoffmann, N. Chem. Rev. 2008, 108, 1052.
(c) Meier, H.; Cao, D. Chem. Soc. Rev. 2013, 42, 143.
/
| 〈 |
|
〉 |