

Chinese Journal of Organic Chemistry >
Synthesis and Bioactivity Evaluation of Acylthiourea Derivatives Based on Isopimaric Acid
Received date: 2016-10-12
Revised date: 2016-12-07
Online published: 2016-12-21
Supported by
Project supported by the National Natural Science Foundation of China (No. 31370575).
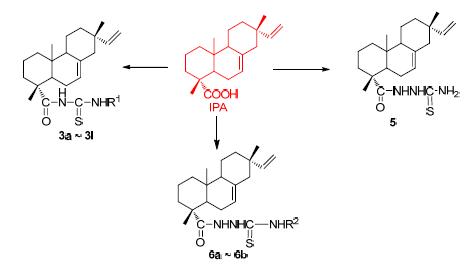
To develop isopimaric acid derivatives with high bioactivity, fifteen acyl (amide) thiourea derivatives containing isopimaric acid skeleton were synthesized and confirmed by FT-IR, 1H NMR, 13C NMR and HRMS or elemental analysis. All the compounds were evaluated for their antibacterial and anticancer activity. Most of the compounds showed siginificant inhibitory activity which was higher than that of isopiamric acid against the Candida Albicans. At the concentration of 100 μmol/L, many compounds exhibited pronounced inhibitory effects on human melanoma cells (A375) and prostatic carcinoma (PC-3) cell lines, especially compounds N-isopimaric acyl-N'-(3-methylphenyl)sulfourea (3c) and N-isopimaric acylamino-N'-(4-fluorophenyl) sulfourea (6b) have the best anticancer activity, both with the inhibitory rate of over 90%.
Liu Juanjuan , Lu Yanju , Wang Jing , Bi Liangwu , Zhao Zhendong . Synthesis and Bioactivity Evaluation of Acylthiourea Derivatives Based on Isopimaric Acid[J]. Chinese Journal of Organic Chemistry, 2017 , 37(3) : 731 -738 . DOI: 10.6023/cjoc201610017
[1] Rabergh, C. M. I.; Lilius, H.; Eriksson, J. E.; Isomaa, B. Aquat. Toxicol. 1999, 46, 55.
[2] Nikinmaa, M.; Wickström, C.; Lilius, H.; Isomaa, B.; Rabergh, C. Environ. Toxicol. Chem. 1999, 18, 993.
[3] Henney, N. C.; Li, B.; Elford, C.; Campbell, A. Am. J. Physiol. Cell Physiol. 2009, 297, C1397.
[4] Wu, C.; Gopal, K. V.; Lukas, T. J.; Gross, G. W.; Moore, E. J. Eur. J. Pharmacology 2014, 732, 68.
[5] Wang, L.; Kang, H.-C.; Li, Y.-Z.; Shui, Y.; Yamamoto, R.; Sugai, T.; Kato, N. Neuropharmacol. 2015, 92, 8.
[6] Sharma, S.; Nagar, V.; Mehta, B. K. Fitoterapia 1993, 64, 476.
[7] Tanaka, R.; Tokuda, H.; Ezaki, Y. Phytomedicine 2008, 15, 985.
[8] Chang, L. C.; Song, L. L.; Park, E. J.; Luyengi, L.; Lee, K. J.; Farnsworth, N. R.; Pezzuto, J. M.; Kinqhorn, A. D. J. Nat. Prod. 2000, 63, 1235.
[9] Adamczyk, S.; Adamczyk, B.; Kitunen, V.; Smolander, A. Soil Biol. Biochem. 2015, 87, 59.
[10] Bisio, A.; Fraternale, D.; Damonte, G.; Millo, E.; Lanteri, A. P.; Russo, E.; Romussi, G.; Parodi, B.; Ricci, D.; De Tommasi, N. Nat. Prod. Commun. 2009, 4, 1621.
[11] Elliger, C. A.; Zinkel, D. F.; Chan, B. G.; Waiss, A. C., Jr. Experientia 1976, 32, 1364.
[12] Perez Gutierrez, R. M.; Garcia, B. E. J. Asian Nat. Prod. Res. 2011, 13, 934.
[13] Cheng, S.-S.; Chang, S.-T. Wood Sci. Technol. 2014, 48, 831.
[14] Xie, Y. S.; Isman, M. B.; Yi, F.; Wong, A. J. Chem. Ecol. 1993, 19, 1075.
[15] Gong, Y.-X.; Wang, Z.-Y.; Zhang, Z.-W.; Chen, C.-B.; Wang, Y.-G. Chin. J. Org. Chem. 2006, 26, 360 (in Chinese).(龚银香, 王子云, 张正文, 陈传兵, 汪炎钢, 有机化学, 2006, 26, 360.)
[16] Zou, X.-J.; Jin, G.-Y.; Yang, Z. Chem. J. Chin. Univ. 2002, 23, 403 (in Chinese). (邹霞娟, 金桂玉, 杨昭, 高等学校化学学报, 2002, 23, 403.)
[17] Su, G.-F.; Huo, L.-N.; Qin, J.-K.; Pan, C.-X.; Guan, Y.-F. Chin. J. Appl. Chem. 2008, 25, 803 (in Chinese).(苏桂发, 霍丽妮, 覃江克, 潘成学, 关一富, 应用化学, 2008, 25, 803.)
[18] Elkholy, S. S.; Salem, H. A.; Eweis, M.; Elsabee, M. Z. Int. J. Biol. Macromol. 2014, 70, 199.
[19] Plutín, A. M.; Mocelo, R.; Alvarez, A.; Ramos, R.; Castellano, E. E.; Cominetti, M. R.; Graminha, A. E.; Ferreira, A. G.; Batista, A. A. J. Inorg. Biochem. 2014, 134, 76.
[20] Koca, ?.; Özgür, A.; Co?kun, K. A.; Tutar, Y. Bioorg. Med. Chem. 2013, 21, 3859.
[21] Wang, Y.-G.; Lu, B.-X.; Ye, W.-F.; Zhao, X.-Y.; Yang, J. Chin. J. Org. Chem. 2002, 22, 862 (in Chinese).(汪焱钢, 卢冰熙, 叶文法, 赵新筠, 杨军, 有机化学, 2002, 22, 862.)
[22] Su, G.-F.; Huo, L.-N.; Chen, R.; Zhao, F.-L.; Guan, Y.-F. Acta Chim. Sinica 2008, 66, 2717 (in Chinese).(苏桂发, 霍丽妮, 陈睿, 赵丰丽, 关一富, 化学学报, 2008, 66, 2717.)
[23] Ulubelen, A.; Oksüz, S.; Topcu, G.; Gören, A. C.; Bozok-Johansson, C.; Celik, C.; Kökdil, G.; Voelter, W. Nat. Pro. Lett. 2001, 15, 307.
[24] Zhao, Z.-D.; Li, X.-D.; Bi, L.-W.; Chen, Y.-X.; Gu, Y.; Li, D.-M.; Wang, J. J. CN 101302151, 2008[Chem. Abstr. 2008, 150, 5919].
[25] Hilliard, N. J.; Duffy, L. B.; Crabb, D. M.; Waites, K. B. J. Microbiol. Methods 2005, 60, 285.
[26] Liu, Z.-J.; Wu, S.-S.; Wang, Y.; Li, R.-J.; Wang, J.; Wang, L.-H.; Zhao, Y.-F.; Gong, P. Eur. J. Med. Chem. 2014, 87, 782.
/
| 〈 |
|
〉 |