

Chinese Journal of Organic Chemistry >
Synthesis and Anti-tumor Activity of 4-(Methoxyl thienyl)-3- (substituted benzoyl)pyrroles
Received date: 2016-10-28
Revised date: 2016-12-02
Online published: 2017-01-04
Supported by
Project supported by the National Science and Technology Major Special Drug Discovery (No. 2010ZX09401404-004).
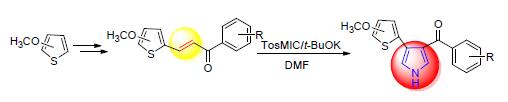
33 novel 4-substituted thienyl pyrrole compounds were synthesized via replacement, Vilsmeier-Hack, aldol condensation and Van Leusen pyrrole reaction using 2-methoxythiophen, 3-methoxythiophene, 3,4-dibromothiophene and substituted acetophenone as raw materials. The structures of all target compounds were characterized by 1H NMR, 13C NMR and HRMS, meanwhile the cell proliferation inhibition efficacy was estimated against CHO, HCT-116, MGC80-3, SGC-7901 and HUVEC cell lines. The results revealed that some target compounds exhibited strong (IC50≤20 μmol/L) or moderate (20 μmol/L < IC50≤50 μmol/L) proliferation inhibition efficacy against tumor cells, meanwhile no significant inhibition activity on HUVEC, which indicated that these compounds had high selectivity. Herein, some compounds showed strong or moderate inhibition efficacy against MGC80-3. The IC50 values of [4-(3,4-dimethoxythiophen-2-yl)-1H-pyrrol-3-yl](4-phenylphenyl)-methanone (4a-2) and [4-(3,4-dimethoxythiophen-2-yl)-1H-pyrrol-3-yl](3-bromophenyl)methanone (4a-7), were 8.6 and 8.5 μmol/L against MGC80-3, respectively, and the IC50 value of 4a-7 was 20.0 μmol/L against HCT-116. Both compounds 4a-2 and 4a-7 exhibited moderate inhibition efficacy against SGC-7901.
Key words: pyrrole; thiophene; synthesis; anti-tumor activity
Zhao Kai , Wang Shuai , Zhan Xiaoping , Liu Zenglu , Mao Zhenmin . Synthesis and Anti-tumor Activity of 4-(Methoxyl thienyl)-3- (substituted benzoyl)pyrroles[J]. Chinese Journal of Organic Chemistry, 2017 , 37(4) : 943 -953 . DOI: 10.6023/cjoc201610044
[1] Gholap, S. S. Eur. J. Med. Chem. 2016, 110, 13.
[2] Estevez, V.; Villacampa, M.; Menendez, J. C. Chem. Soc. Rev. 2010, 39, 4402.
[3] Ma, J.; Lu, X.; Xia, Y.; Yan, F. J. Chromatogr. Sci. 2015, 53, 380.
[4] Lazerges, M.; Chane-Ching, K. I.; Aeiyach, S.; Chelli, S.; PeppinDonnat, B.; Billon, M.; Lombard, C.; Maurel, F.; Jouini, M. J. Solid State Electrchem. 2009, 13, 231.
[5] Walsh, C. T.; Garneau-Tsodikova S.; Howard-Jones, A. R. Nat. Prod. Rep. 2006, 23, 517.
[6] Battilocchio, C.; Poce, G.; Alfonso, S. Bioorg. Med. Chem. 2013, 21, 3695.
[7] Biava, M.; Porretta, G. C.; Deidda, D.; Pompei, R.; Tafic A.; Manettic, F. Bioorg. Med. Chem. 2004, 12, 1453.
[8] Protopopova, M.; Bogatcheva, E.; Nikonenko, B.; Hundert, S.; Einck, L.; Nacy, C. A. Med. Chem. 2007, 3, 301.
[9] Shattat, G. F.; Abuskeika, G. M.; Al-Qirim, T. M. Lat. Am. J. Pharm. 2015, 34, 1258.
[10] Dannhardt, G.; Kiefer, W.; Krämer, G.; Maehrlein, S.; Nowe U.; Fiebich, B. Eur. J. Med. Chem. 2000, 35, 499.
[11] Teixeira, C.; Barbault, F.; Rebehmed, J.; Liu, K.; Xie, L.; Lu, H.; Jiang, S.; Fan B.; Maurel, F. Bioorg. Med. Chem. 2008, 16, 3039.
[12] Lankheet, N. A. G.; Hillebrand, M. J. X.; Rosing H.; Schellens, J. H. M.; Beijnen, J. H.; Huitema, A. D. R. Biomed. Chromatogr. 2013, 27, 466.
[13] Molina, A. M.; Jia, X.; Feldman, D. R. Clin. Genitourin. Cancer 2013, 11, 297.
[14] Bailly, C. Mar. Drugs 2015, 13, 1105.
[15] Lan, L.; Zhan, X.-P.; Qin, W.-X.; Liu, Z.-L.; Mao, Z.-M. Heterocycles 2014, 89, 375.
[16] Lan, L.; Qin, W.-X.; Zhan, X.-P.; Liu, Z.-L.; Mao, Z.-M. Anti-Cancer Agents Med. Chem. 2014, 14, 994.
[17] Li, Y.-Z.; Zhao, P.; Zhan, X.-P.; Liu, Z.-L.; Mao, Z.-M. Chin. J. Org. Chem. 2015, 35(1), 167 (in Chinese).
(李衍忠, 赵萍, 詹晓平, 刘增路, 毛振民, 有机化学, 2015, 35(1), 167.)
[18] Zhan, X.-P.; Lan, L.; Zhang, Y.-K.; Chen, J.; Zhao, K.; Wang, S.; Xin, Y.-X.; Mao, Z.-M. Bull. Korean Chem. Soc. 2016, 37, 200.
[19] Zhan, X.-P.; Lan, L.; Wang, S. Chem. Biodiversity 2016, 14(2), n/a.
[20] Chen, J.; Zhang, Y.-K.; Zhan, X.-P.; Liu, Z.-L.; Mao, Z.-M. Chin. J. Org. Chem. 2016, 36, 572 (in Chinese).
(陈简, 张袁魁, 詹晓平,刘增路, 毛振民, 有机化学, 2016, 36, 572.)
[21] Chen, S.; Lu, B. Y.; Duan, X. J. Polym. Sci. Part A: Polym. Chem. 2012, 50, 1967.
[22] Shen, P.; Liu, X. P.; Jiang, S. H.; Wang, L.; Yi, L.; Ye, D. D.; Zhao, B.; Tan, S. T. Dyes Pigm. 2012, 92, 1042.
[23] Vilsmeier, A.; Haack, A. Chem. Ber. 1927, 60, 119
[24] Blockhuys, F.; Hoefnagels, R.; Peten, C.; Alsenoy, C. V.; Geise, H. J. J. Mol. Struct. 1999, 485, 87.
[25] Batista, R. M. F.; Costa, S. P. G.; Belsley, M.; Lodeiro, C.; Raposo, M. M. M. Tetrahedron 2008, 64, 9230.
[26] van Leusen, A. M.; Siderius, H.; Hoogenboom, B. E.; van Leusen, D. Tetrahedron Lett. 1972, 52, 5337.
[27] Leeper, F. J.; Kelly, J. M. Org. Prep. Proced. Int. 2013, 45, 171.
[28] Zhou, J. J; Yue, X. F.; Han, J. X.; Yang, W. Y. Chin. J. Pharm. 1993, 24, 455.
[29] Chadwick, J. J. Chem. Soc., Perkin Trans. 1 1973, 2327.
/
| 〈 |
|
〉 |