

Chinese Journal of Organic Chemistry >
Recent Advances in 3-Isothiocyanato Oxindoles Engaged Asymmetric Cascade Reactions
Received date: 2016-11-15
Revised date: 2016-12-21
Online published: 2017-01-04
Supported by
Project supported by the National Natural Science Foundation of China (No. 21602052) and the Scientific Research Project of Hubei Provincial Department of Education (No. Q20163004).
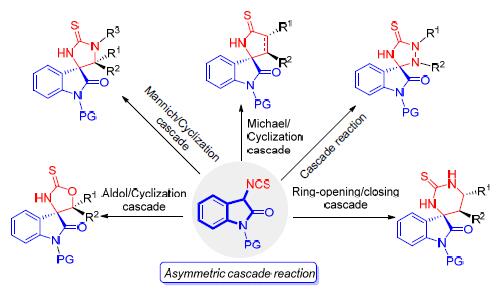
3-Isothiocyanato oxindoles have been widely employed as a class of highly reactive and novel reagents in the enantioselective synthesis of diverse spirooxindoles. This review summarizes the recent advances of 3-isothiocyanato oxindoles mediated some types of cascade process in the past six years, including properties of reaction, activation models and synthetic applications. Furthermore, the prospects of this concept are also discussed.
Tan Fen , Xiao Wenjing , Zeng Guoping . Recent Advances in 3-Isothiocyanato Oxindoles Engaged Asymmetric Cascade Reactions[J]. Chinese Journal of Organic Chemistry, 2017 , 37(4) : 824 -840 . DOI: 10.6023/cjoc201611017
[1] Lin, H.; Danishefsky, S. J. Angew. Chem., Int. Ed. 2003, 42, 36.
[2] Marti, C.; Carreira, E. M. Eur. J. Org. Chem. 2003, 2209.
[3] (a) Zhou, F.; Liu, Y.-L.; Zhou, J. Adv. Synth. Catal. 2010, 352, 1381.
(b) Yu, J.; Shi, F.; Gong, L.-Z. Acc. Chem. Res. 2011, 44, 1156.
(c) Rios, R. Chem. Soc. Rev. 2012, 41, 1060.
(d) Cheng, D.-J.; Ishihara, Y.; Tan, B.; Barbas III, C. F. ACS Catal. 2014, 4, 743.
(e) Xiao, Y.-L.; Zhou, Y.; Wang, J.; Wang, J.-X.; Liu, H. Chin. J. Org. Chem. 2015, 35, 2035 (in Chinese).
(肖永龙, 周宇, 王江, 王进欣, 柳红, 有机化学, 2015, 35, 2035.)
[4] Suchy, M.; Kutschy, P.; Monde, K. J. Org. Chem. 2001, 66, 3940.
[5] (a) Cui, C. B.; Kakeya, H.; Osada, H. J. Antibiot. 1996, 49, 832.
(b) Edmondson, S.; Danishefsky, S.-J.; Sepp-Lorenzino, L.; Rosen, N. J. Am. Chem. Soc. 1999, 121, 2147.
(c) Ding, K.; Lu, Y.; Nikolovska-Coleska, Z.; Qiu, S.; Ding, Y.; Gao, W.; Stuckey, J.; Krajewski, K.; Roller, P. P.; Tomita, Y.; Parrish, D. A.; Deschamps, J. R.; Wang, S. J. Am. Chem. Soc. 2005, 127, 10130.
(d) Cheng, M.-N.; Wang, H.; Gong, L.-Z. Org. Lett. 2011, 13, 2418.
[6] Jossang, A.; Jossang, P.; Hadi, H. A.; Sevenet, T.; Bodo, B. J. Org. Chem. 1991, 56, 6527.
[7] (a) Potawel, S. E.; Mehta, U. K.; Waseem, S.; Dhalawat, H. J.; Lunya, K. P.; Mantri, R. A.; Vetol, Y. D. Pharmacology 2008, 2, 197.
(b) Litvinov, Y. M.; Mortikov, V. Y.; Shestopalov, A. M. J. Comb. Chem. 2008, 10, 741.
[8] Rottmann, M.; McNamara, C.; Yeung, B. K. S.; Lee, M. C. S.; Zou, B.; Russell, B.; Seitz, P.; Plouffe, D. M.; Dharia, N. V.; Tan, J.; Cohen, S. B.; Spencer, K. R.; González-Páez, G. E.; Lakshminarayana, S. B.; Goh, A.; Suwanarusk, R.; Jegla, T.; Schmitt, E. K.; Beck, H. P.; Brun, R.; Nosten, F.; Renia, L.; Dartois, V.; Keller, T. H.; Fidock, D. A.; Winzeler, E. A.; Diagana, T. T. Science 2010, 329, 1175.
[9] Liang, H.; Li, R.-M.; Yuan, Q.-P. J. Beijing Univ. Chem. Technol. (Nat. Sci.) 2015, 42, 1 (in Chinese).
(梁浩, 李瑞敏, 袁其朋, 北京化工大学学报(自然科学版), 2015, 42, 1.)
[10] (a) Chen, W.-B.; Wu, Z.-J.; Hu, J.; Cun, L.-F.; Zhang, X.-M.; Yuan, W.-C. Org. Lett. 2011, 13, 2472.
(b) Han, W.-Y.; Zhao, J.-Q.; Zuo, J.; Xu, X.-Y.; Zhang, X.-M.; Yuan, W.-C. Adv. Synth. Catal. 2015, 357, 3007.
[11] Jiang, K.; Jia, Z.-J.; Yin, X.; Wu, L.; Chen, Y.-C. Org. Lett. 2010, 12, 2766.
[12] Han, Y.-Y.; Chen, W.-B.; Han, W.-Y.; Wu, Z.-J.; Zhang, X.-M.; Yuan, W.-C. Org. Lett. 2012, 14, 490.
[13] Kayal, S.; Mukherjee, S. Org. Lett. 2015, 17, 5508.
[14] Chen, W.-B.; Han, W.-Y.; Han, Y.-Y.; Zhang, X.-M.; Yuan, W.-C. Tetrahedron 2013, 69, 5281.
[15] (a) Kato, S.; Kanai, M.; Matsunaga, S. Chem. Asian J. 2013, 8, 1768.
(b) Kato, S.; Kanai, M.; Matsunaga, S. Heterocycles 2014, 88, 475.
[16] Kato, S.; Yoshino, T.; Shibasaki, M.; Kanai, M.; Matsunaga, S. Angew. Chem., Int. Ed. 2012, 51, 7007.
[17] (a) Vassilev, L. T.; Vu, B. T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C.; Fotouhi, N.; Liu, E. A. Science 2004, 303, 844.
(b) Tovar, C.; Rosinski, J.; Filipovic, Z.; Higgins, B.; Kolinsky, K.; Hilton, H.; Zhao, X.; Vu, B. T.; Qing, W.; Packman, K.; Myklebost, O.; Heimbrook, D. C.; Vassilev, L. T. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 1888.
[18] Shangary, S.; Qin, D.; McEachern, D.; Liu, M.; Miller, R. S.; Qiu, S.; Nikolovska-Coleska, Z.; Ding, K.; Wang, G.; Chen, J.; Bernard, D.; Zhang, J.; Lu, Y.; Gu, Q.; Shah, R. B.; Pienta, K. J.; Ling, X.; Kang, S.; Guo, M.; Sun, Y.; Yang, D.; Wang, S. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 3933.
[19] Cai, H.; Zhou, Y.; Zhang, D.; Xu, J.-Y.; Liu, H. Chem. Commun. 2014, 50, 14771.
[20] Bai, M.; Cui, B.-D.; Zuo, J.; Zhao, J.-Q.; You, Y.; Chen, Y.-Z.; Xu, X.-Y.; Zhang, X.-M.; Yuan, W.-C. Tetrahedron 2015, 71, 949.
[21] Du, D.; Xu, Q.; Li, X.-G.; Shi, M. Chem. Eur. J. 2016, 22, 4733.
[22] Cao, Y.-M.; Shen, F.-F.; Zhang, F.-T.; Wang, R. Chem. Eur. J. 2013, 19, 1184.
[23] Wu, H.; Zhang, L.-L.; Tian, Z.-Q.; Huang, Y.-D.; Wang, Y.-M. Chem. Eur. J. 2013, 19, 1747.
[24] Tan, F.; Cheng, H.-G.; Feng, B.; Zou, Y.-Q.; Duan, S.-W.; Chen, J.-R.; Xiao, W.-J. Eur. J. Org. Chem. 2013, 2071.
[25] Wu, S.; Zhu, X.-L.; He, W.-J.; Wang, R.-M.; Xie, X.-H.; Qin, D.-B.; Jing, L.-H.; Chen, Z.-Q. Tetrahedron 2013, 69, 11084.
[26] Tan, F.; Lu, L.-Q.; Yang, Q.-Q.; Guo, W.; Bian, Q.; Chen, J.-R.; Xiao, W.-J. Chem. Eur. J. 2014, 20, 3415.
[27] Fu, Z.-K.; Pan, J.-Y.; Xu, D.-C.; Xie, J.-W. RSC Adv. 2014, 4, 51548.
[28] Kayal, S.; Mukherjee, S. Eur. J. Org. Chem. 2014, 6696.
[29] (a) Zhao, J.-Q.; Zhou, M.-Q.; Wu, Z.-J.; Wang, Z.-H.; Yue, D.-F.; Xu, X.-Y.; Zhang, X.-M.; Yuan, W.-C. Org. Lett. 2015, 17, 2238.
(b) Zhao, J.-Q.; Wu, Z.-J.; Zhou, M.-Q.; Xu, X.-Y.; Zhang, X.-M.; Yuan, W.-C. Org. Lett. 2015, 17, 5020.
[30] Du, D.; Jiang, Y.; Xu, Q.; Shi, M. Adv. Synth. Catal. 2013, 355, 2249.
[31] Wang, L.-Q.; Yang, D.-X.; Li, D.; Liu, X.-H.; Zhao, Q.; Zhu, R.-R.; Zhang, B.-Z.; Wang, R. Org. Lett. 2015, 17, 4260.
[32] Du, D.; Jiang, Y.; Xu, Q.; Tang, X.-Y.; Shi, M. ChemCatChem 2015, 7, 1366.
[33] Chowdhury, R.; Kumar, M.; Ghosh, S. K. Org. Biomol. Chem. 2016, 14, 11250.
[34] Liu, X.-L.; Han, W.-Y.; Zhang, X.-M.; Yuan, W.-C. Org. Lett. 2013, 15, 1246.
[35] Han, W.-Y.; Li, S.-W.; Wu, Z.-J.; Zhang, X.-M.; Yuan, W.-C. Chem. Eur. J. 2013, 19, 5551.
[36] Chen, Q.; Liang, J.-Y.; Wang, S.-L.; Wang, D.; Wang, R. Chem. Commun. 2013, 49, 1657.
[37] Cui, B.-D.; Li, S.-W.; Zuo, J.; Wu, Z.-J.; Zhang, X.-M.; Yuan, W.-C. Tetrahedron 2014, 70, 1895.
[38] Zhao, H.-W.; Tian, T.; Pang, H.-L.; Li, B.; Chen, X.-Q.; Yang, Z.; Meng, W.; Song, X.-Q.; Zhao, Y.-D.; Liu, Y.-Y. Adv. Synth. Catal. 2016, 358, 2619.
[39] Kayal, S.; Mukherjee, S. Org. Biomol. Chem. 2016, 14, 10175.
[40] Liu, L.; Zhao, B.-L.; Du, D.-M. Eur. J. Org. Chem. 2016, 4711.
[41] Jiang, Y.; Pei, C.-K.; Du, D.; Li, X.-G.; He, Y.-N.; Xu, Q.; Shi, M. Eur. J. Org. Chem. 2013, 7895.
[42] Wang, L.-Q.; Yang, D.-X.; Li, D.; Wang, R. Org. Lett. 2015, 17, 3004.
[43] Zhu, G.-M.; Sun, W.-S.; Wu, C.-Y.; Li, G.-F.; Hong, L.; Wang, R. Org. Lett. 2013, 15, 4988.
/
| 〈 |
|
〉 |