

Chinese Journal of Organic Chemistry >
Synthesis of Bis-naphthalene and Their Derivatives and Their Complexation with Organic Cation
Received date: 2016-12-08
Revised date: 2016-12-27
Online published: 2017-01-04
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21302090, 21572097) and the Thousand Talents Program-Youth.
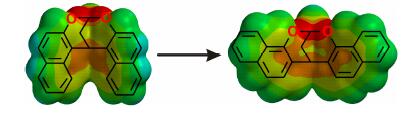
In this research, the structure of the bis-naphthalene molecular resulting from 2-naphthol and 1,1,1',1'-tetramethoxypropane was modified, and six derivatives with different substituents, sidewalls or bridges were synthesized. Their structures were studied by X-ray crystallography and molecular modelling, and all possess curved architectures. Electrostatic potential energy surfaces show that their inner cavities are electron-rich, and may complex with organic cations through cation-π interactions. Their binding stoichiometry and association constants with the 1,4-diazabicyclo[2.2.2]octane (DABCO)-based organic cation were studied by 1H NMR titration and Job's plot. The results show that electron-rich molecules have much stronger association, and reducing the carbon atom in the bridge significantly decreases their association ability. These novel curved structures may work as building blocks for the construction of new macrocyclic receptors.
Key words: host-guest chemistry; macrocyclic chemistry; bis-naphthalene
Yao Huan , Sun Jiaonan , Ke Hua , Yang Liupan , Li Jiarong , Jiang Wei . Synthesis of Bis-naphthalene and Their Derivatives and Their Complexation with Organic Cation[J]. Chinese Journal of Organic Chemistry, 2017 , 37(3) : 603 -607 . DOI: 10.6023/cjoc201612033
[1] (a) Tian, J.; Chen, L.; Zhang, D. W.; Liu, Y.; Li, Z. T. Chem. Commun. 2016, 52, 6351.
(b) Chi, X.; Yu, G.; Shao, L.; Chen, J.; Huang, F. J. Am. Chem. Soc. 2016, 138, 3168.
(c) Gao, B.; Tan, L. L.; Song, N.; Li, K.; Yang, Y. W. Chem. Commun. 2016, 52, 5804.
(d) Zhang, W.; Zhang, Y. M.; Li, S. H.; Cui, Y. L.; Yu, J.; Liu, Y. Angew. Chem., Int. Ed. 2016, 128, 11624.
(e) Ma, J.; Meng, Q.; Hu, X.; Li, B.; Ma, S.; Hu, B.; Li, C. Org. Lett. 2016, 18, 5740.
(f) Chen, H.; Fan, J.; Hu, X.; Ma, J.; Wang, S.; Li, J.; Li, C. Chem. Sci. 2015, 6, 197.
(g) Wang, X.; Han, K.; Li, J.; Jia, X.; Li, C. Polym. Chem. 2013, 4, 3998.
(h) Wang, Y.; Ping, G.; Li, C. Chem. Commun. 2016, 52, 9858.
[2] (a) Zhou, C. E.; Zhao, Z. G.; Tang, X. L. Chin. J. Org. Chem. 2007, 27, 513 (in Chinese).(周彩娥, 赵志刚, 唐晓丽, 有机化学, 2007, 27, 513.)
(b) Peng, Y.; Mou, Q. M.; Yang, Z. X.; Chen, S. H. Chin. J. Org. Chem. 2004, 24, 399 (in Chinese).(彭游, 牟其明, 杨祖幸, 陈淑华, 有机化学, 2004, 24, 399.)
[3] (a) Rebek, J. Science 1987, 235, 1478.
(b) Rebek, J. Pure Appl. Chem. 1989, 61, 1517.
[4] Harmata, M. Acc. Chem. Res. 2004, 37, 862.
[5] Dolenský, B.; Havlík, M.; Král, V. Chem. Soc. Rev. 2012, 41, 3839.
[6] (a) Han, T.; Chen, C. F. Org. Lett. 2006, 8, 1069.
(b) Chen, C. F. Chem. Commun. 2011, 47, 1674.
(c) Jiang, Y.; Chen, C. F. Eur. J. Org. Chem. 2011, 32, 6377.
(d) Meng, Z.; Han, Y.; Wang, L. N.; Xiang, J. F.; He, S. G.; Chen, C. F. J. Am. Chem. Soc. 2015, 137, 9739.
[7] Yang, L. P.; Liu, W. E.; Jiang, W. Tetrahedron Lett. 2016, 57, 3978.
[8] (a) Jia, F.; He, Z.; Yang, L. P.; Pan, Z. S.; Yi, M.; Jiang, R. W.; Jiang, W. Chem. Sci. 2015, 6, 6731.
(b) Jia, F.; Wang, H. Y.; Li, D. H.; Yang, L. P.; Jiang, W. Chem. Commun. 2016, 52, 5666.
(c) Jia, F.; Li, D. H.; Yang, T. L.; Yang, L. Pan.; Dang, L.; Jiang, W. Chem. Commun. 2017, 53, 336.
(d) Yang, L. P.; Jia, F.; Zhou, Q. H.; Pan, F.; Sun, J. N.; Rissanen, K.; Chung, L. W.; Jiang, W. Chem.-Eur. J. 2017, 23, 1516.
[9] He, Z.; Yang, X.; Jiang, W. Org. Lett. 2015, 17, 3880.
[10] (a) He, Z.; Ye, G.; Jiang, W. Chem. Eur. J. 2015, 21, 3005.
(b) Huang, G. B.; Jiang, W. Prog. Chem. 2015, 27, 744 (in Chinese).(黄国宝, 蒋伟, 化学进展, 2015, 27, 744.)
[11] (a) Huang, G.; He, Z.; Cai, C. X.; Pan, F.; Yang, D.; Rissanen, K.; Jiang, W. Chem. Commun. 2015, 51, 15490.
(b) Huang, G.; Valkonen, A.; Rissanen, K.; Jiang, W. Chem. Commun. 2016, 52, 9078.
[12] Huang, G. B.; Wang, S. H.; Ke, H.; Yang, L. P.; Jiang, W. J. Am. Chem. Soc. 2016, 138, 14550.
[13] Van Allan, J. A.; Giannini, D. D.; Whitesides, T. H. J. Am. Chem. Soc. 1982, 47, 820.
[14] Shorthill, B. J.; Avetta, C. T.; Glass, T. E. J. Am. Chem. Soc. 2004, 126, 12732.
[15] Geiseler, O.; Müller, M.; Podlech, J. Tetrahedron 2013, 69, 3683.
[16] Kito, T.; Yoshinaga, K.; Yamaye, M.; Mizobe, H. J. Org. Chem. 1991, 56, 3336.
/
| 〈 |
|
〉 |