

Chinese Journal of Organic Chemistry >
Simple Synthesis of 5E-Decen-1-ol and 5E-Decenyl Acetate, the Sex Pheromone of Peach Twig Borer
Received date: 2016-08-18
Revised date: 2016-11-12
Online published: 2017-01-10
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21362018, 81460525) and the Applied Basic Research Project of Yunnan Province (No. 2014FB117).
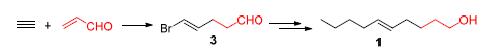
Peach twig borer is one of the most serious pests, whose sex pheromone is 5E-decen-1-ol and 5E-decenyl acetate. Synthesis of both compounds was achieved from acrolein. Firstly, a tandem addition reaction of acrolein with acetylene in the presence of Pd(OAc)2 gave 5-bromo-4E-pentenal, which reacted with the ylide prepared from (methoxymethyl)triphenyl- phodphonium chloride to afford (1E,5E/Z)-1-bromo-6-methoxyhexadiene. The compound was coupled with Grignard reagent, followed by hydrolysis and reduction to give 5E-decen-1-ol. Esterification of the alcohol afforded 5E-decenyl acetate.
Huang Fei , Yang Wanqiu , Zhang Yushun , Yao Yun , Tao Yunhai . Simple Synthesis of 5E-Decen-1-ol and 5E-Decenyl Acetate, the Sex Pheromone of Peach Twig Borer[J]. Chinese Journal of Organic Chemistry, 2017 , 37(4) : 1046 -1050 . DOI: 10.6023/cjoc201608011
[1] Bai, J.-W.; Zhao, J.-X.; Ma, W.-L. Sci. Silvae Sin. 1980, S1, 127 (in Chinese).
(白九维, 赵剑霞, 马文梁, 林业科学, 1980, S1, 127.)
[2] (a) Meng, X.-Z. Entomol. Knowl. 2000, 37, 75 (in Chinese).
(孟宪佐, 昆虫知识, 2000, 37, 75.)
(b) Meng, X.-Z. Chin. Bull. Entomol. 2007, 44, 477 (in Chinese).
(苏茂文, 张钟宁, 昆虫知识, 2007, 44, 477.)
[3] Ni, C.-C. World Pestic. 2008, 30, 31 (in Chinese).
(倪长春, 世界农药, 2008, 30, 31.)
[4] Roelofs, W. L.; Kochansky, J.; Anthon, E.; Rice, R.; Carde, R. Environ. Entomol. 1975, 4, 580.
[5] (a) Ohloff, G.; Vial, C.; Näf, F.; Pawlak, M. Helv. Chim. Acta 1977, 60, 1161.
(b) Odinokov, V. N.; Balezina, G. G.; Ishmuratov, G. Y.; Vakhitov, R. S.; Tolstikov, G. A. Chem. Nat. Compd. 1985, 21, 369.
(c) Poleschner, H.; Heydenreich, M.; Martin, D. Synthesis 1991, 1231.
(d) Doolittle, R. E.; Patrick, D. G.; Heath, R. H. J. Org. Chem. 1993, 58, 5063.
[6] (a) Buss, A. D.; Greeves, N.; Mason, R.; Warren, S. J. Chem. Soc., Perkin Trans. 1 1987, 2569.
(b) Levin, D.; Warren, S. Tetrahedron Lett. 1985, 26, 505.
(c) Buss, A. D.; Greeves, N.; Levin, D.; Wallace, P.; Warren, S. Tetrahedron Lett. 1984, 25, 357.
[7] Vig, O. P.; Sharma, M. L.; Gakhar, M.; Malik, N. Indian J. Chem., Sect. B 1980, 19, 755.
[8] Miyano, S.; Hokari, H.; Umeda, Y.; Hashimoto, H. Bull. Chem. Soc. Jpn. 1980, 53, 770.
[9] Chattopadhyay, A.; Mamdapur, V. R.; Chadha, M. S. Indian J. Chem., Sect. B 1983, 22, 1221.
[10] Zakharkin, L. I.; Petrushkina, E. A. Bull. Acad. Sci. USSR, Div. Chem. Sci. 1985, 34, 193.
[11] Chauret, D. C.; Chong, J. M. Tetrahedron Lett. 1993, 34, 3695.
[12] Pederson, R. L.; Grubbs, R. H. US 20020022741, 2002 [Chem. Abstr. 2002, 143315].
[13] (a) Tao, Y.-H.; Cheng, W.-X.; Zhang, Y.-S.; Gu, K. Chem. J. Chin. Univ. 2005, 26, 1072 (in Chinese).
(陶云海, 程伟贤, 张玉顺, 古昆, 高等学校化学学校, 2005, 26, 1072.)
(b) Tao, Y.-H.; Cheng, W.-X.; Huang, X.-Z.; Zhang, Y.-S.; Gu, K. Chin. J. Org. Chem. 2005, 25, 558 (in Chinese).
(陶云海, 程伟贤, 黄相中, 张玉顺, 古昆, 有机化学, 2005, 25, 558.)
(c) Ma, Z.; Yang X.; Zhang, Y.; Huang, X.; Tao, Y. Synlett 2012, 23, 581.
(d) Tao, Y.; Yang, X.; Jin, Y.; Wang, Q. Synth. Commun. 2013, 43, 415.
(e) Yang, X.-M.; Zhang, Y.-Z.; Yao, Y.; Tao, Y.-H. Chin. J. Org. Chem. 2014, 34, 1458 (in Chinese).
(杨晓梅, 张玉顺, 姚赟, 陶云海, 有机化学, 2014, 34, 1458.)
[14] Wang, Z.; Lu, X.; Lei, A.; Zhang, Z. J. Org. Chem. 1998, 63, 3806.
/
| 〈 |
|
〉 |