

Chinese Journal of Organic Chemistry >
One-Pot Synthesis of Trifluoromethylated Homoallylic N-Acylhydrazines Promoted by Indium Powder
Received date: 2016-11-04
Revised date: 2016-12-26
Online published: 2017-01-10
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21262031, 21462037).
An one-pot reaction of trifluoroacetaldehyde methyl hemiacetal or trifluoroketones, acylhydrazines and allyl bromide promoted by indium powder is reported, and a series of trifluoromethylated homoallylic N-acylhydrazines were obtained with good to excellent yields. The features of this process include readily available starting materials as trifluoromethyl building blocks, mild conditions and easy operation.
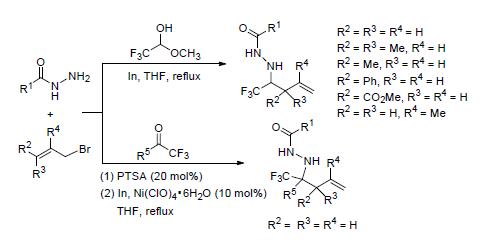
Li Jun , Yang Tianyu , Zhang Huaiyuan , Huang Danfeng , Wang Kehu , Su Yingpeng , Hu Yulai . One-Pot Synthesis of Trifluoromethylated Homoallylic N-Acylhydrazines Promoted by Indium Powder[J]. Chinese Journal of Organic Chemistry, 2017 , 37(4) : 925 -935 . DOI: 10.6023/cjoc201611009
[1] (a) Mishra, M.; Tiwari, K.; Mourya, P. Polyhedron 2015, 89, 29.
(b) Aubin, S.; Martin, B.; Delcros, J. G. J. Med. Chem. 2005, 48, 330.
(c) Huang, R.; Wang, Q. J. Orgnomet. Chem. 2001, 637, 94.
(d) Ke, S.; Sun, T.; Liang, Y. Yang, Z. Chin. J. Org. Chem. 2010, 30, 1820 (in Chinese).
(柯少勇, 孙婷婷, 梁英, 杨自文, 有机化学, 2010, 30, 1820.)
[2] (a) Zheng, L. W.; Wu, L. L.; Zhao, B. X.; Dong, W. L.; Miao, J. Y. Bioorg. Med. Chem. 2009, 17 1957.
(b) Lian, S.; Su, H.; Zhao, B. X.; Liu, W. Y.; Zheng, L. W.; Miao, J. Y. Bioorg. Med. Chem. 2009, 17, 7085.
[3] Duarte, C. D.; Tributino, J. L. M.; Lacerda, D. I.; Martins, M. V.; Alexandre-Moreira, M. S.; Dutra, F.; Bechara, E. J. H.; De-Paula, F. S.; Goulart, M. O. F.; Ferreira, J.; Calixto, J. B.; Nunes, M. P.; Bertho, A. L.; Miranda, A. L. P.; Barreiroa, E. J.; Fraga, C. A. M. Bioorg. Med. Chem. 2007, 15, 2421.
[4] (a) Formicola, L.; Maréchal, X.; Basse, N.; Bouvier-Durand, M.; Bonnet-Delpon, D.; Milcent, T.; Reboud-Ravaux, M.; Ongeri, S. Bioorg. Med. Chem. 2009, 19, 83.
(b) Onnis, V.; Cocco, M. T.; Fadda, R.; Congiu, C. Bioorg. Med. Chem. 2009, 17, 6158.
[5] (a) Wing, K. D. Science 1988, 241, 467.
(b) Wing, K. D.; Slawecki, R. A.; Carlson, G. R. Science 1988, 241, 470.
[6] (a) Yoneya, M.; Takada, S.; Maeda, Y.; Yokoyama, H. Liq. Cryst. 2008, 35, 339.
(b) Parra, M.; Hidalgo, P.; Barberá, J.; Carrasco, E.; Saavedra, C. Liq. Cryst. 2006, 33, 391.
(c) Cui, H.; Xu, Y.; Zhang, Z. F. Anal. Chem. 2004, 76, 4002.
[7] (a) Licandro, E.; Perdicchia, D. Eur. J. Org. Chem. 2004, 665.
(b) Flagstad, T.; Petersen, M. T.; Nielsen, T. E. Angew. Chem., Int. Ed. 2015, 54, 8395.
(c) Liu, Z.; Zhao, J.; Huang, X. Bioorg. Med. Chem. Lett. 2006, 16, 1828.
(d) Bechara, W. S.; Khazhieva, I. S.; Rodriguez, E.; Charette, A. B. Org. Lett. 2015, 17, 1184.
(e) Gao, Q.; Liu, S.; Wu, X.; Zhang, J.; Wu, A. Org. Lett. 2015, 17, 2960.
(f) Lavergne, K.; Bongers, A.; Betit, L.; Beauchemin, A. M. Org. Lett. 2015, 17, 3612.
[8] (a) Alonso, C.; Marigorta, E. M.; Rubiales, G.; Palacios, F. Chem. Rev. 2015, 115, 1847.
(b) Tomashenko, O. A.; Grushin, V. V. Chem. Rev. 2011, 111, 4475.
(c) Ma, J.-A.; Cahard, D. Chem. Rev. 2008, 108, PR1.
(d) Qing, F. Chin. J. Org. Chem. 2012, 32, 815 (in Chinese).
(卿凤翎, 有机化学, 2012, 32, 815.)
[9] (a) Fustero, S.; Simên-Fuentes, A.; Barrio, P.; Haufe, G. Chem. Rev. 2015, 115, 871.
(b) Nie, J.; Guo, H.-C.; Cahard, D.; Ma, J.-A. Chem. Rev. 2011, 111, 455.
(c) Schlosser, M. Angew. Chem., Int. Ed. 2006, 45, 5432.
(d) Uneyama, K.; Katagiri, T.; Amii, H. Acc. Chem. Res. 2008, 41, 817.
[10] (a) Shen, Z.-L.; Wang, S.-Y.; Chok, Y.-K.; Xu, Y.-K.; Loh, T.-P. Chem. Rev. 2013, 113, 271.
(b) Roy, U. K.; Roy, S. Chem. Rev. 2010, 110, 2472.
(c) Zhao, K.; Shen, L.; Shen, Z.-L.; Loh, T.-P. Chem. Soc. Rev. 2017, 46, 586.
[11] Issac, M. B.; Chan, T.-H. Tetrahedron Lett. 1995, 36, 8957.
[12] Kornblum, N.; Chen, S. I.; Kelly, W. J. J. Org. Chem. 1988, 53, 1831.
[13] Chen, W. J.; Liao, D. H. Chem. World 2006, (5), 285 (in Chinese).
(陈文杰, 廖道华, 化学世界, 2006, (5), 285.)
/
| 〈 |
|
〉 |